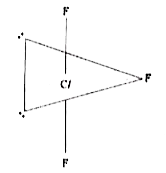
Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|9 VideosCHEMICAL BONDING AND MOLECULAR STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|9 VideosATOMIC STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|22 VideosCHEMICAL EQUILIBRIUM AND ACIDS-BASES
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|8 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CHEMICAL BONDING AND MOLECULAR STRUCTURE-SHORT ANSWER QUESTIONS
- Show how a double and triple bond are formed between carbon atoms in ...
Text Solution
|
- Show how a double and triple bond are formed between carbon atoms in ...
Text Solution
|
- Explain hybridisation of phosphorous in the formation of PCl(5)
Text Solution
|
- Explain the hybridisation involved is SF(6).
Text Solution
|
- Explain the formation of Coordinate Covalent bond with one example.
Text Solution
|
- Which hybrid orbitals are used by Carbon atoms in the following molecu...
Text Solution
|
- Which hybrid orbitals are used by Carbon atoms in the following molecu...
Text Solution
|
- Which hybrid orbitals are used by Carbon atoms in the following molecu...
Text Solution
|
- Which hybrid orbitals are used by Carbon atoms in the following molecu...
Text Solution
|
- Explain different types of hydrogen bonds with examples.
Text Solution
|
- Explain the formation H(2) molecule on the basis of Valence Bond theor...
Text Solution
|
- Using Molecular Orbital Theory explain why the B(2) molecule is parama...
Text Solution
|
- Write the important conditions necessary for linear combination of ato...
Text Solution
|
- What is meant by the term Bond order? Calculate the bond orders in the...
Text Solution
|
- Of BF(3) " and " NF(3), dipolemoment is observed for NF(3) and not for...
Text Solution
|
- Even though both NH(3) " and " NF(3) are pyramidal, NH(3) has a higher...
Text Solution
|
- How do you predict the shapes of the following molecules making use of...
Text Solution
|
- How do you predict the shapes of the following molecules making use of...
Text Solution
|
- How do you predict the shapes of the following molecules making use of...
Text Solution
|
- How do you predict the shapes of the following molecules making use of...
Text Solution
|