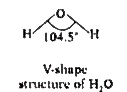Text Solution
Verified by Experts
Topper's Solved these Questions
CHEMICAL BONDING AND MOLECULAR STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise SHORT ANSWER QUESTIONS|33 VideosATOMIC STRUCTURE
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|22 VideosCHEMICAL EQUILIBRIUM AND ACIDS-BASES
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|8 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-CHEMICAL BONDING AND MOLECULAR STRUCTURE-LONG ANSWER QUESTIONS
- Explain the formation of Ionic Bond with a suitable example.
Text Solution
|
- Explain the factors favourable for the formation of Ionic Compounds.
Text Solution
|
- Draw Lewis Structures for the following molecules. (a) H(2)S " ...
Text Solution
|
- Write notes on (a) Bond Angle (b) Bond Enthalpy (c)Bond length and (d)...
Text Solution
|
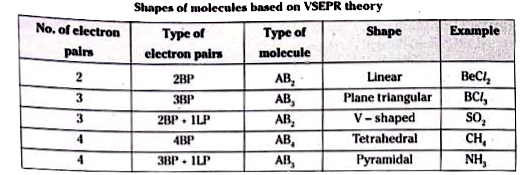
- Write about VSEPR theory.
Text Solution
|
- Explain the formation of BF3 molecule with the help of Valency Bond th...
Text Solution
|
- What do you understand by Hybridisation ? Explain different types of h...
Text Solution
|
- Write the salient features of Molecular Orbital Theory.
Text Solution
|
- Give the Molecular Orbital Energy diagram of (a) N(2) and (b) O(2). Ca...
Text Solution
|