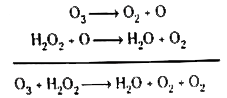
Text Solution
Verified by Experts
Topper's Solved these Questions
STOICHIOMETRY
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTION|13 VideosSTOICHIOMETRY
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|10 VideosSTOICHIOMETRY
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTIONS & ANSWERS|10 VideosSTATES OF MATTER : GASES AND LIQUIDS
VGS PUBLICATION-BRILLIANT|Exercise ADDITIONAL QUESTION & ANSWERS|10 VideosTHE P-BLOCK ELEMENTS - GROUP 13
VGS PUBLICATION-BRILLIANT|Exercise LONG ANSWER QUESTIONS|3 Videos
Similar Questions
Explore conceptually related problems
VGS PUBLICATION-BRILLIANT-STOICHIOMETRY-SHORT ANSWER QUESTIONS
- Suggest a list of the substances where carbon exhibit oxidation states...
Text Solution
|
- While sulphue dioxide and hydrogen peroxide and act as oxidising as we...
Text Solution
|
- Consider the reactions a) 6CO(2)(g)+6H(2)O(I)toC(6)H(12)O(6)(aq)+6O(...
Text Solution
|
- The compound AgF(2) is unstable compound. However, if formed, the comp...
Text Solution
|
- Whenever a reaction between an oxidising agent and a reducing agent is...
Text Solution
|
- How do you count the following observations? a) Though alkaline pota...
Text Solution
|
- Identify the substance oxidised, reduced, oxidising agent and reducing...
Text Solution
|
- Consider the reactions 2S(2)O(3)^(2-)(aq)+I(2)(s)toS(4)O(6)^(2-)(aq)...
Text Solution
|
- Justify giving reactions that among halogens, fluorine is the best oxi...
Text Solution
|
- Why does the following reaction occur ? XeO(6)^(4-)(aq)+2F^(-)(aq)+6...
Text Solution
|
- Consider the reactions : a) H(3)PO(2)(aq)+4AgNO(3)(aq)+2H(2)O(l)toH(...
Text Solution
|
- Balance the following redox reaction in basic medium by ion-electron m...
Text Solution
|
- Balance the following equations in basic medium by ion-electron method...
Text Solution
|
- What sorts of information can you draw from the following reaction ? ...
Text Solution
|
- The Mn^(3+) ion is unstable solution and undergoes disproportionation ...
Text Solution
|
- Consider the elements Cs, Ne, I and F. a) Identify the element that ...
Text Solution
|
- Chlorine is used to purify drinking water. Excess of Chlorine is harmf...
Text Solution
|
- Refer to the periodic table given in your book and now answer the foll...
Text Solution
|
- In Ostwal's process for the manufacture of nitric acid the first step ...
Text Solution
|
- i) Arrange the following metals in the order in which they displace ea...
Text Solution
|