A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
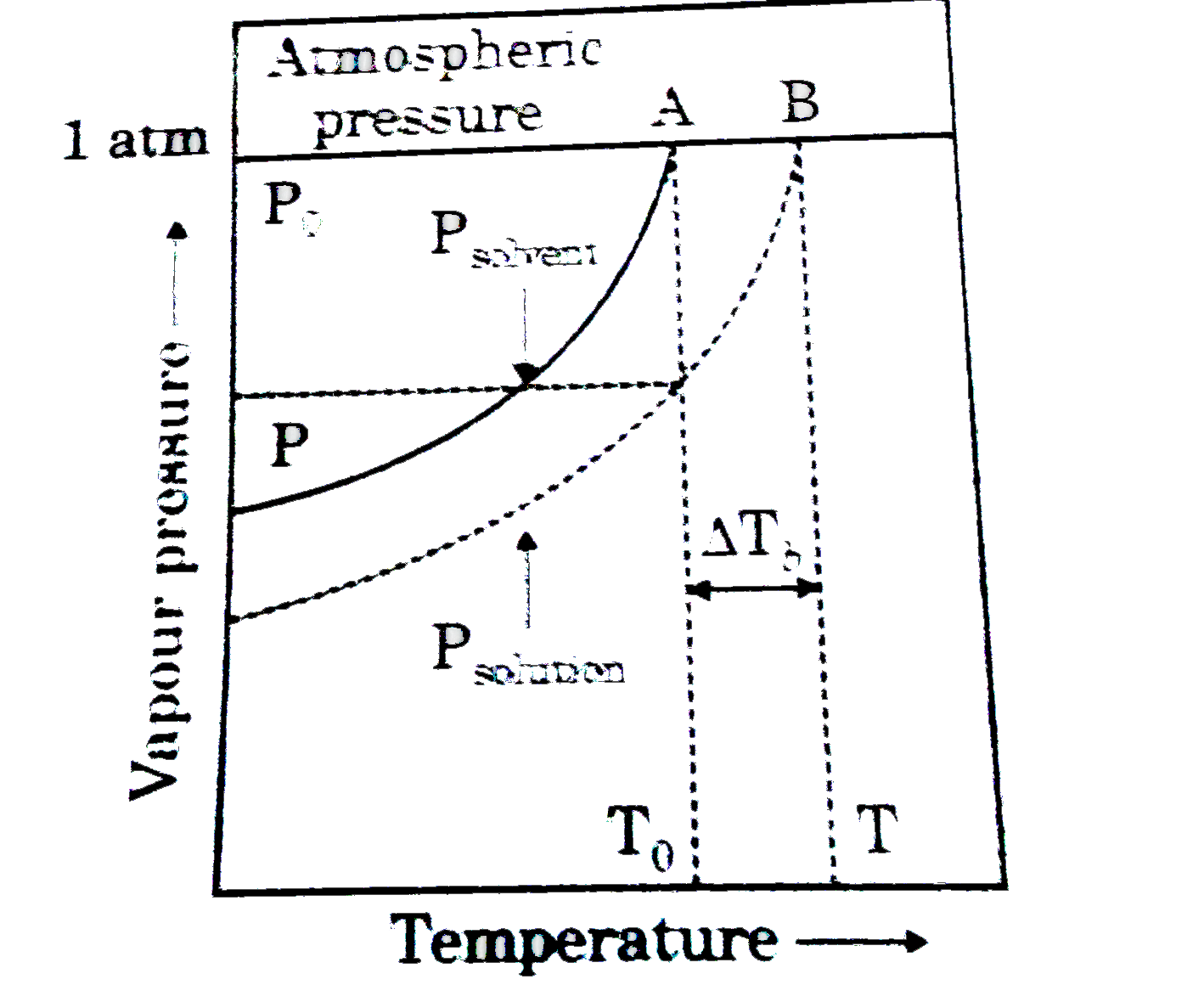
- Figure explains elevation in boiling point when a non-volatile solute ...
Text Solution
|
- Elevation in boiling point of an aqueous solution of urea is 0.52 ( k(...
Text Solution
|
- Elevation in b.p. of an aqueous urea solution is 0.52^(@), (K(b)=0.622...
Text Solution
|
- An aqueous solution of 0.01 M KCl cause the same elevation in boiling ...
Text Solution
|
- Figure explains elevation in boiling point when a non-volatile solute ...
Text Solution
|
- Figure explains elevation in boiling point when a non-volatile solute ...
Text Solution
|
- An aqueous solution of urea is found to boil at 100.52^(@)C . Given K(...
Text Solution
|
- एक जलीय विलयन यूरिया के क्वथनांक में उन्नयन 0.52^(@) है। [K(b) = 0.52^...
Text Solution
|
- Consider following figure and answer the questions at the end of it. F...
Text Solution
|