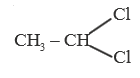A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- H-C equiv C - H + 2HCl rarrFinal product, final product will be:
Text Solution
|
- Me - -=-H overset(NaNH(2))underset(+liq.NH(3))rarr (C ) overset(H(3)O^...
Text Solution
|
- What is the final product of reaction ? CH(3)-CH(2)-C equiv CHoverset(...
Text Solution
|
- Identify final product In the following reaction CH(3)-underset(OH)und...
Text Solution
|
- The final product 'C' in the above reaction is
Text Solution
|
- H-C-=C-H underset(H(2)SO(4))overset(HgSO(4))(rarr)overset("dil.NaOH")(...
Text Solution
|
- The final product C obtained in this reaction
Text Solution
|
- H-C equiv C - H + 2HCl rarr Final product, final product will be:
Text Solution
|
- H-C equiv C - H + 2HCl rarrFinal product, final product will be:
Text Solution
|