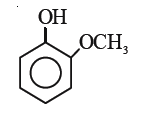
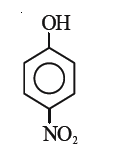
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Phenol is more acidic than :
Text Solution
|
- Phenols are much more acidic than alcohols. Explain.
Text Solution
|
- Phenol is more acidic than
Text Solution
|
- समझाइए की क्यों ऑर्थोनाइट्रो फीनॉल, ऑर्थो मेथॉक्सी फीनॉल से अधिक अम्ल...
Text Solution
|
- फिनॉल्स के अम्लीय व्यवहार की व्याख्या कीजिए। फिनॉल्स, ऐल्कॉहॉल्स की तु...
Text Solution
|
- कार्बोक्सिलक अम्ल्, फीनॉल की अपेक्षा अधिक अम्लीय होता है। समझाइए।
Text Solution
|
- Phenols are more acidic than aliphatic alcohols because
Text Solution
|
- Phenol is more acidic than :
Text Solution
|
- निम्नलिखित के कारण बतायें - (a) फेनॉल इथेनॉल से अधिक अम्लीय है। (b...
Text Solution
|