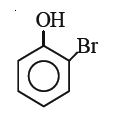
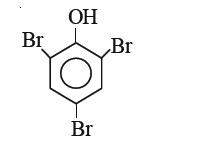
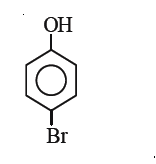
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- The product of the reaction of phenol with bromine water is :
Text Solution
|
- Explain why phenol with bromine water forms 2,4,6-tribromophenol while...
Text Solution
|
- Explain why phenol with bromine water forms 2,4,6-tribromophenol while...
Text Solution
|
- फीनॉल ब्रोमीन जल के साथ क्रिया करके त्रिविस्थपित उत्पाद बनाता ह...
Text Solution
|
- When phenol is treated with excess of bromine water ,the product forme...
Text Solution
|
- Phenol on treatment with bromine water gives
Text Solution
|
- With bromine water phenol gives
Text Solution
|
- The product of the reaction of phenol with bromine water is :
Text Solution
|
- The product of the reaction of phenol with bromine water is :
Text Solution
|