Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
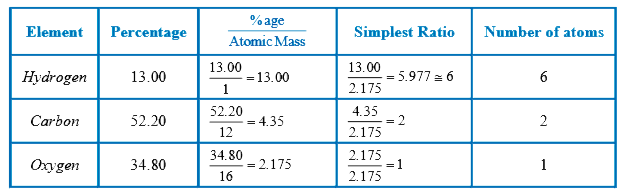
- A compound contains 34.8% Oxygen, 52.2% carbon and 13.0% Hydrogen. Wha...
Text Solution
|
- 64g of an organic compound contains 24 g of carbon, 8gm of hydrogen an...
Text Solution
|
- In a compound Carbond =52.2% , Hydrogen =13%, Oxygen =34.8% are presen...
Text Solution
|
- Each 9.4gm of a compound contains 7.2gm carbon, 0.6gm hydrogen and res...
Text Solution
|
- An organic compound contains 40% carbons atom 6.67% hydrogen atoms and...
Text Solution
|
- A compound contains 34.8% oxygen, 52.2% carbon and 13.0% hydrogen. Wha...
Text Solution
|
- A compound of carbon, hydrogen and oxygen contains 40% of carbon and 6...
Text Solution
|
- An organic compound, consisting of carbon, hydrogen, nitrogen and oxyg...
Text Solution
|
- A compound contains 34.8% Oxygen, 52.2% carbon and 13.0% Hydrogen. Wha...
Text Solution
|