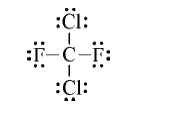Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- Write a Lewis structure for "CC"I(2)F(2) one of the compounds indicate...
Text Solution
|
- Write a Lewis structure for "CC"I(2)F(2) one of the compounds indicate...
Text Solution
|
- एक रासायनिक यौगिक का नाम लिखे जिससे ओजोन स्तर का अवक्षय होता है।
Text Solution
|
- Write the effects of ozone depletion.
Text Solution
|
- Write the names & structure of following compounds : An organic compou...
Text Solution
|
- Write one effect of depletion of ozone layer and one measure for the p...
Text Solution
|
- Write an essay an ozone depletion.
Text Solution
|
- एल्किन के ओजोनिकरण द्वारा कार्बोनिल योगिक प्राप्त करने का समीकरण लिखिए...
Text Solution
|
- Write the full name of the group of compounds mainly responsible for t...
Text Solution
|