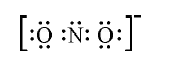Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
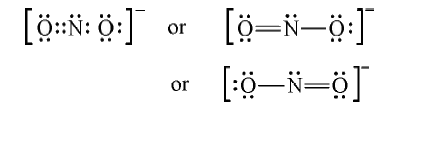
- Write the Lewis dot structure of the nitrite ion (NO(2)^(Θ)) .
Text Solution
|
- Write the Lewis dot structure of the nitrite ion (NO(2)^(Θ)) .
Text Solution
|
- Write the Lewis dot structure of CO(3)^(2-) ion .
Text Solution
|
- Write the Lewis structure for CN^(Θ) ion .
Text Solution
|
- Write the resonance structure of NO(2)^(Θ) (nitrite) and NO(3)^(Θ) (ni...
Text Solution
|
- Write the various steps involved in the Lewis structure for nitrate (N...
Text Solution
|
- Write the Lewis structure of nitrite ion, No(2)- .
Text Solution
|
- The Lewis dot structure of carbonate ion is …………..
Text Solution
|
- नाइट्राइट आयन, NO(2) के लिए 'लूइस संरचना' लिखें |
Text Solution
|