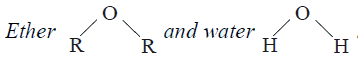Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- have same hybridization at oxygen yet they have different bond angles....
Text Solution
|
- Assertion: Bond angle at oxygen in ethehers is greater than ideal tetr...
Text Solution
|
- In both water and dimethyl ether (CH(3)-underset(* *)overset(* *)O-CH...
Text Solution
|
- In both water and dimethyl ether (CH(3)-underset(..)overset(..)O-CH(3)...
Text Solution
|
- The bond angle formed by different hybrid orbitals are in the order
Text Solution
|
- The bond angle formed by different hybrid orbitals are in the order
Text Solution
|
- In both water and dimethyl ether (CH(3)-underset(..)overset(..)O-CH(3)...
Text Solution
|
- In both water and diethyl ether, the central atom viz. O-atoms has sam...
Text Solution
|
- have same hybridization at oxygen yet they have different bond angles....
Text Solution
|