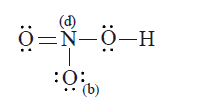Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
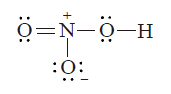
- Calculate formal charge (F) on nitrogen and Oxygen atoms marked 'a' an...
Text Solution
|
- In the following electron dot structure, calculate the formal charge f...
Text Solution
|
- The incorrect set of the formal charge on different atoms in the Lewis...
Text Solution
|
- Which is corrent Lewis structure with formal charge on particular atom...
Text Solution
|
- In Lewis structure of ozone (O(3)) , formal charges on all three oxyge...
Text Solution
|
- Calculate formal charge (F) on nitrogen and Oxygen atoms marked 'a' an...
Text Solution
|
- The Lewis structure for O(3) molecule is given below. The correct fo...
Text Solution
|
- Calculate the formal charge on each oxygen atom in ozone.
Text Solution
|
- The formal charge on the carbon atom in the following structure unders...
Text Solution
|