Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
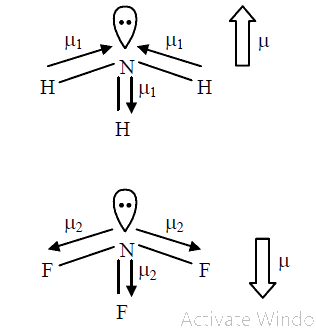
- Dipole moment of NH(3) is more than that of NF(3), Explain.
Text Solution
|
- Dipole moment of NH(3) isthan NF(3) .
Text Solution
|
- The dipoles moment of NF(3) is less than NH(3) because
Text Solution
|
- NH(3) का द्विध्रव आघूर्ण NF(3) से अधिक होता है ।
Text Solution
|
- Assertion : Dipole moment of NH(3) is greater than that of NF(3). Re...
Text Solution
|
- Although F is more electronegative than H , then resultant dipole mome...
Text Solution
|
- Dipole moment of NF(3) is smaller than :
Text Solution
|
- Dipole moment of NH(3) is more than that of NF(3), Explain.
Text Solution
|
- Out of NH(3) and NF(3) which is more polar. Explain with the help of d...
Text Solution
|