A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
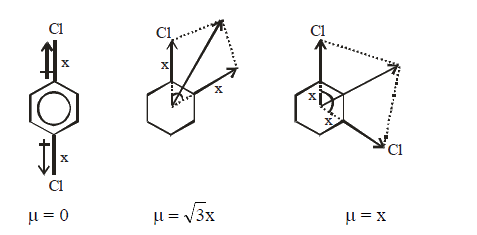
- The dipole moment of o, p and m-dichlorobenzene will be in the order :
Text Solution
|
- The dipole moment of o, p and m-dichlorobenzene will be in the order :
Text Solution
|
- Four compounds, Toluene (I), o-dichlorobenzene (II), m-dichlorobenzene...
Text Solution
|
- The dipole moment of o, p and m-dichlorobenzene will be in the order :
Text Solution
|
- Arrange the following compounds in order of increasing dipole moment, ...
Text Solution
|
- निम्न यौगिकों को उनके द्विध्रुव आघूर्ण के बढ़ते हुए क्रम में व्यवस्थित ...
Text Solution
|
- टॉलुईन (I), m- डाइक्लोरोबेन्जीन (II), o- डाइक्लोरोबेन्जीन (III) तथ...
Text Solution
|
- The dipole moment of o, m and p-dichlorobenzene will be in the order :
Text Solution
|
- Arrange the following compounds in order of increasing dipole moment :...
Text Solution
|