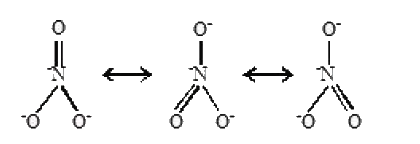
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Similar Questions
Explore conceptually related problems
Recommended Questions
- Bond order of N-O bonds in nitrate ion is
Text Solution
|
- The correct order of N-O bond length is
Text Solution
|
- Bond order of N-O bonds in nitrate ion is
Text Solution
|
- The bond order in He2^(+) ions is :
Text Solution
|
- Cl-O bond order in perchlorate ion is
Text Solution
|
- The bond order in O2^- ion is
Text Solution
|
- The bond order of the N-O bonds in NO3^- ion is
Text Solution
|
- The O-N-O bond angle in the nitrite ion, NO(2)^(-), is closest to :
Text Solution
|
- The O-N-O bond angles in the nitrate ion, NO(3)^(-) are best described...
Text Solution
|