Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
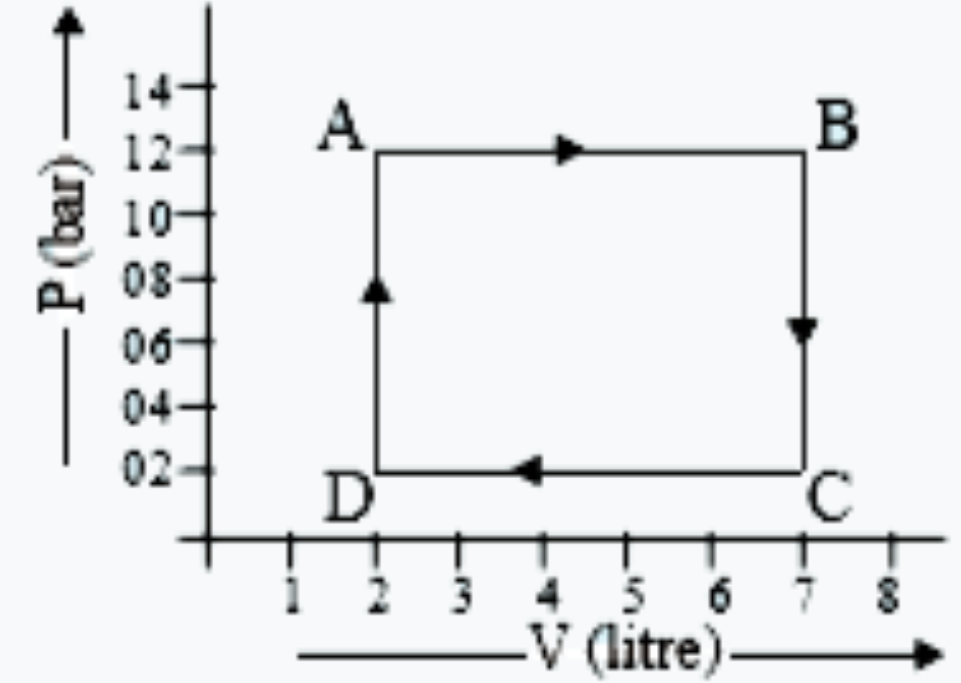
- The diagram shows a P - V graph of a thermodynamic behaviour of an ide...
Text Solution
|
- An ideal gas undergoes a cyclic process A to B to C to D for which P –...
Text Solution
|
- The diagram shows a PV graph of a thermodynamic behavious of an ideal ...
Text Solution
|
- In the adjoining diagram, the p-V graph of an ideal gas is shown. Find...
Text Solution
|
- संलग्न चित्र में, एक आदर्श गैस की ऊष्मागतिकीय प्रक्रियाओं का P-V। ग्रा...
Text Solution
|
- संलग्न चित्र में, एक आदर्श गैस की ऊष्मागतिकीय प्रक्रियाओं का P-V। ग्रा...
Text Solution
|
- चित्र में, किसी गैस के लिए दाब-आयतन आरेख (P-V diagram) दर्शावा गया है...
Text Solution
|
- चित्र में, किसी गैस के लिए दाब-आयतन आरेख (P-V diagram) दर्शाया गया है...
Text Solution
|
- चित्र में, किसी गैस के लिये दाय-आयतन आरेख दर्शाया गया है। ग्राफ से ज्...
Text Solution
|