Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
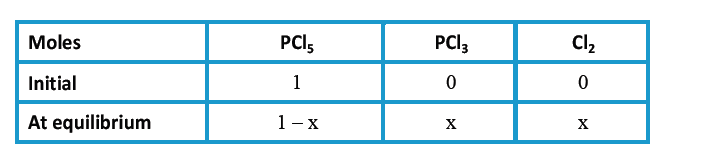
- When PCl(5) is heated, it dissociates into PCl(3) and Cl(2). The vapou...
Text Solution
|
- When PCl(5) is heated, it dissociates into PCl(3) and Cl(2) . The vapo...
Text Solution
|
- The vapour density of PCl(5) at 43K is is found to be 70.2 . Find the ...
Text Solution
|
- The vapour density of Pcl(5) is 104.16 but when heated to 230^(@)C, it...
Text Solution
|
- For the dissociation of PCl(5) into PCl(3) and Cl(2) in gaseous phase ...
Text Solution
|
- At 200^(@)C PCl(5) dissociates as follows : PCl(5)(g0hArrPCl(3)(g)+Cl(...
Text Solution
|
- What is the vapour density of mixture of PCL(5) at 250^(@)C when it ha...
Text Solution
|
- The vapour density of PCl(5) at 473 is found to be 70*2 . Find the deg...
Text Solution
|
- The Vapour Density of mixture of PCl(5), PCl(3) and Cl(2) is 92. Find ...
Text Solution
|