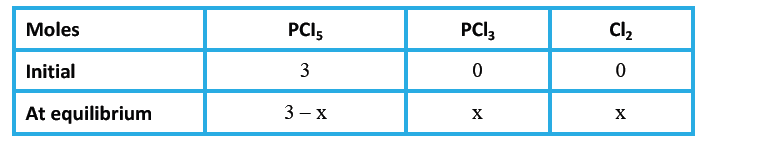
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- 1mol of Cl(2) and 3 mol of PCl(5) are placed in a 100 L vessel heated ...
Text Solution
|
- In the dissociation of PCl(5) as PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) If ...
Text Solution
|
- 1 mol of Cl(2) and 3 mol of PCl(5) are placed in a 100 L vessel heated...
Text Solution
|
- 1 mole of PCl(5) taken at 5 atm, dissociates into PCl(3) and Cl(2) to...
Text Solution
|
- PCl(5)(g) hArr PCl(3)(g)+Cl(2)(g) 'alpha' is the degree of dissociatio...
Text Solution
|
- a' moles of PCl(5) are heated in a closed container to equilibrate PCl...
Text Solution
|
- PCl(3),Cl(2),PCl(5) are in equilibrium in a closed vessel at 500K. The...
Text Solution
|
- PCl(5)(g)hArr PCl(3)(g)+Cl(2)(g),K(p)=1.8. At 250^(@)C if 50% of PCl(5...
Text Solution
|
- Find out the value of K(p)//K(c) for the reaction PCl(5)(g)hArr PCl(3)...
Text Solution
|