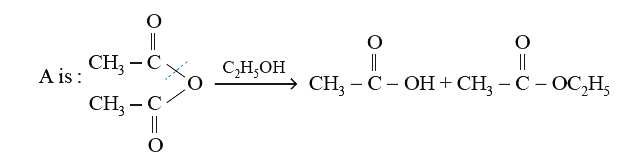Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- An organic compound (A) on treatment with ethyl alcohol gives a carbox...
Text Solution
|
- An organic compound (A) on treatment with ethyl alcohol gives a carbox...
Text Solution
|
- Hydrolysis of an ester gives acid A and alcohol B. The acid reduces Fe...
Text Solution
|
- An organic compound 'A' on treatment with ethyl alcohol gives a carbox...
Text Solution
|
- An organic compound (A) reacts with ethanol to give (B) and (C). On ac...
Text Solution
|
- एक कार्बनिक यौगिक (A) ऐथिल ऐल्कोहॉल के साथ क्रिया करके एक कार्बाक्सिलि...
Text Solution
|
- An organic compound (A) on treatment with ethyl alcohol gives a carbox...
Text Solution
|
- An organic compound A of molecular formula C(3)H(6)O on reduction with...
Text Solution
|
- An organic compound (A) of molecular formula C2H3n on acid hydrolysis ...
Text Solution
|