A
B
C
D
Text Solution
Verified by Experts
Similar Questions
Explore conceptually related problems
Recommended Questions
- The correct order of the basicities of the following compounds is CH(...
Text Solution
|
- Write the IUPAc name of the following compound : (a) CH(3)-CH(2)-ov...
Text Solution
|
- Which of the following is correct order of basic strength for the give...
Text Solution
|
- The correct order of basicities of the following compounds is , " "und...
Text Solution
|
- Write basicty order of following : CH(3)-underset((P))overset(NH)ove...
Text Solution
|
- CH(3)-underset(OH)underset(|)overset(CH(3))overset(|)(C)-underset(NH(2...
Text Solution
|
- The correct order of basicities of the following compounds is - (I) , ...
Text Solution
|
- CH(3)-underset(CH(3))underset(|)overset(CH(3))overset(|)C-NH(2)एक 3^(@...
Text Solution
|
- Write the commons of the following: CH(3)-CH(2)-CH(2)-underset(CH(3))u...
Text Solution
|
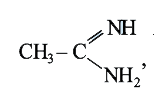 `CH_(3)-underset(2)(CH_(2))-NH_(2)-underset(3)((CH_(3))_(2)-NH,CH_(3)-underset(4)overset(O)overset(||)(C)-NH_(2)`
`CH_(3)-underset(2)(CH_(2))-NH_(2)-underset(3)((CH_(3))_(2)-NH,CH_(3)-underset(4)overset(O)overset(||)(C)-NH_(2)` More basic due to presence of two basic poles.
More basic due to presence of two basic poles.  `gt(CH_(3))_(2)underset(3)(NH)gtCH_(3)-underset(2)(CH_(2)NH_(2)gtunderset(4)(CH_(3)CONH_(2))`
`gt(CH_(3))_(2)underset(3)(NH)gtCH_(3)-underset(2)(CH_(2)NH_(2)gtunderset(4)(CH_(3)CONH_(2))`