A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-STRUCTURE OF THE ATOM-Structure Of The Atom
- Rutherford's alpha-particle scattering experiment showed that (i) el...
Text Solution
|
- The ion of an element has 3 positive charges. Mass number of the atom ...
Text Solution
|
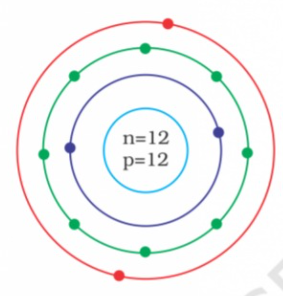
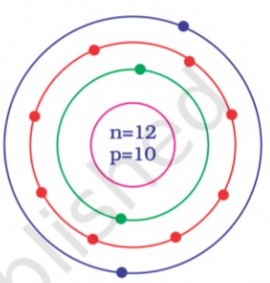
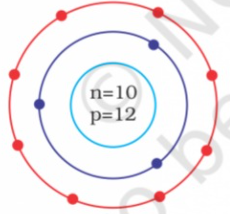
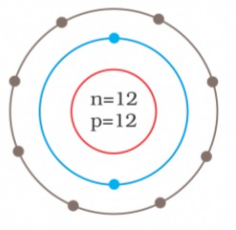
- Identify the Mg^(2+) ion from the figure where, n and p represent the ...
Text Solution
|
- In a sample of ethyl ethanote (CH(3)COOC(2)H(5)) the two oxygen atoms ...
Text Solution
|
- Elements with valency 1 are
Text Solution
|
- The first model of an atom was given by
Text Solution
|
- An atom with 3 protons and neutrons will have a valency of
Text Solution
|
- The electron distribution in an aluminium atom is
Text Solution
|
- Which of the following in figure do not represent Bohr's model of an a...
Text Solution
|
- Which of the following statement is always correct?
Text Solution
|
- Atomic models have been improved over the years. Arrange the following...
Text Solution
|
- Is it possible for the atom of an element to have one electron, one pr...
Text Solution
|
- Write any two observation which support the fact that atoms are divisi...
Text Solution
|
- Will ""^(35)Cl and ""^(37)Cl have different valencies? Justify your an...
Text Solution
|
- Why did Rutherford select a gold foil in his alpha-ray scattering expe...
Text Solution
|
- Find out the valency of the atoms represented by the Figure (a) and (b...
Text Solution
|
- One electron is present in the outermost shell of the atom of an eleme...
Text Solution
|
- Write down the electron distribution of chlorine atom. How many electr...
Text Solution
|
- In the atom of an element X, 6 electrons are present in the outermost ...
Text Solution
|
- What information do you get from the figure about the atomic number, m...
Text Solution
|