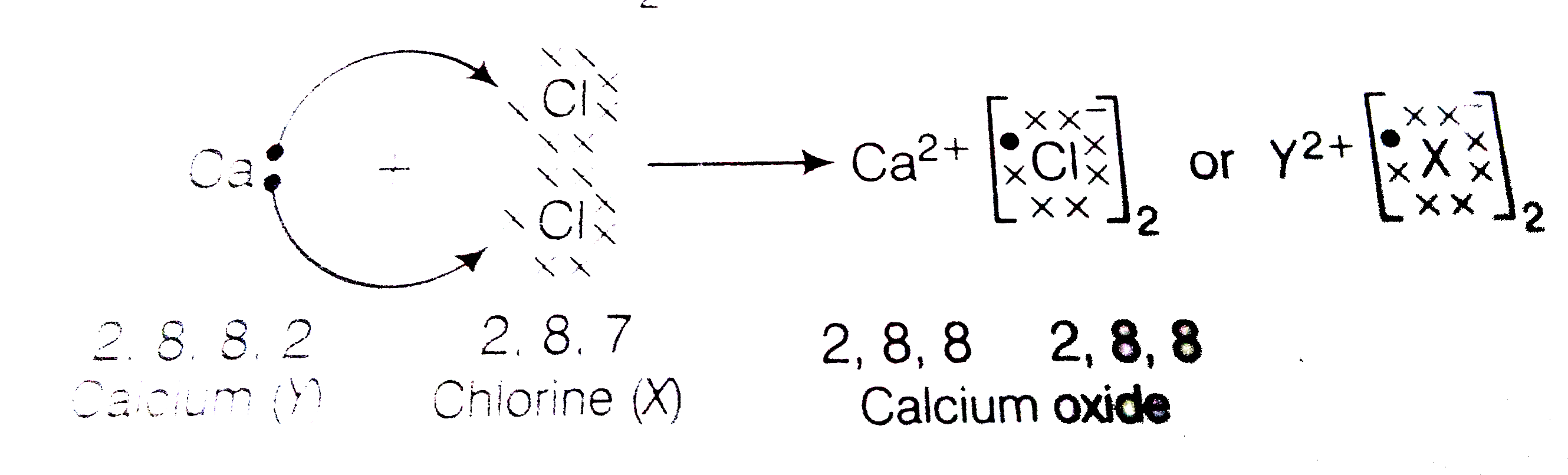Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-PERIODIC CLASSIFICATION OF ELEMENTS-Periodic Classification Of Elements
- Three elements A, B and C have 3,4 and 2 electrons respectively in the...
Text Solution
|
- If an element X is placed in group 14, what will be the formula and th...
Text Solution
|
- Compare the radii of two species X and Y. Give reasons for your answer...
Text Solution
|
- Arrangement the following elements in increasing order of their atomic...
Text Solution
|
- Identify and name the metal out of the following elements whose electr...
Text Solution
|
- Write the formula of the product formed when the element A (atomic num...
Text Solution
|
- Arrange the following elements in the increasing order of their metall...
Text Solution
|
- Identify, the elements with the following property and arrange them in...
Text Solution
|
- Properties of the elements are given below. Where would you locate the...
Text Solution
|
- An element is placed in 2^("nd") group and 3^("rd") period of the peri...
Text Solution
|
- An element X (atomic number 17) reacts with an element Y (atomic numbe...
Text Solution
|
- Atomic number of few elements are given below 10, 20, 7, 14 (a) Iden...
Text Solution
|
- Complete the following crossword puzzle (Figure) Across (1) An ele...
Text Solution
|
- (a) In this ladder (Figure) symbols of elements are jumbled up. Rearra...
Text Solution
|
- Mendeleev predicted the existence of certain elements not known at tha...
Text Solution
|
- (a) Electropositive nature of the element(s) increases down the group ...
Text Solution
|
- An element X which is a yellow solid at room temperature shows catenat...
Text Solution
|
- An element X of group 15 exists as diatomic molecule and combines with...
Text Solution
|
- Which group of elements could be placed in mendeleev's periodic table ...
Text Solution
|
- Give an account of the process adopted by Mendeleev for the classifica...
Text Solution
|