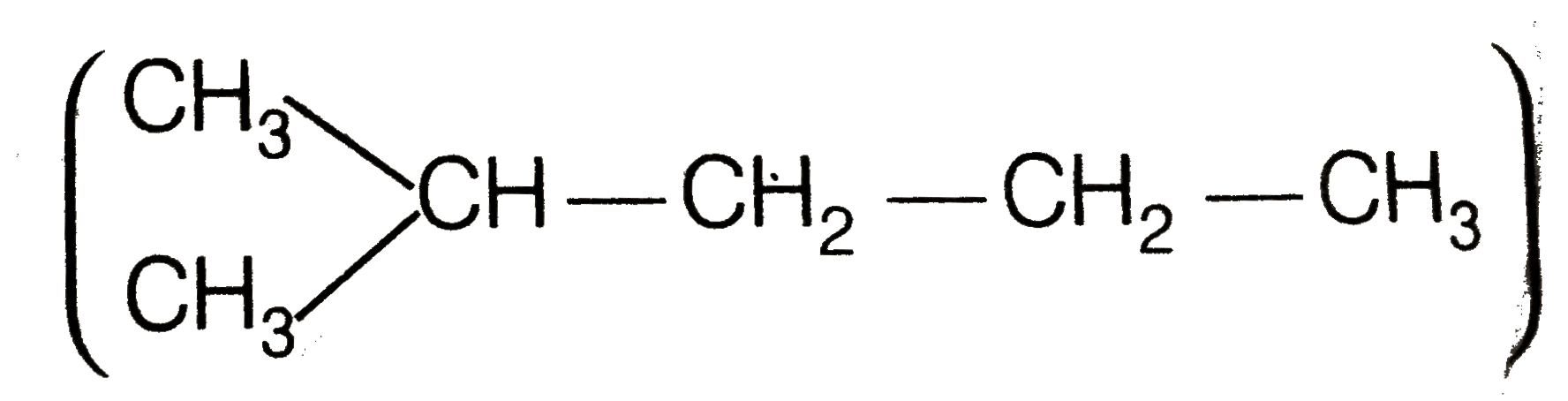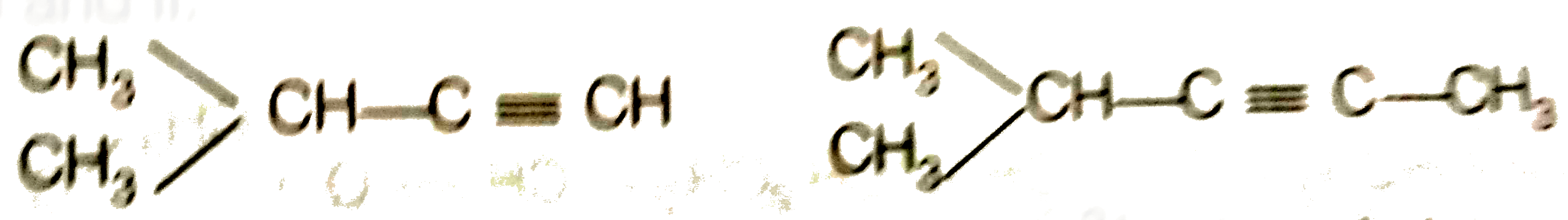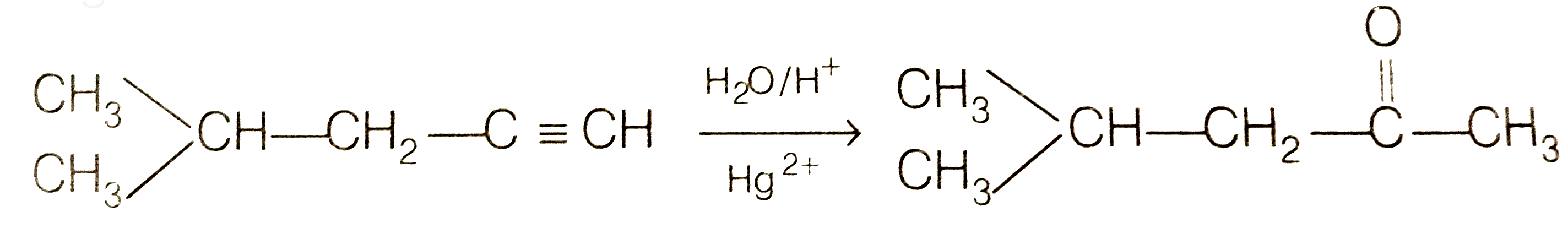To determine the molecular mass of hydrocarbon (A) 896 mL vapour of `C_(x)H_(y)` (A) weighs `3.28` g at STP
22700 mL vapour of `C_(x)H_(y)` (A) weighs `(328xx22700)/(896)g//`"mol at" STP
= `83.1g//mol`
Hence , molecular mass of `C_(x)H_(y)` (A) = `83.1g mol^(-)` To determine the empirical formula of hydrocarbon (A)
`{:("Elament" ,%,"Atomic mass","Relative ratio","Relative no. of atoms","Somplest ratio"),(C,87.8,12,7.31,1,3),(H,12.19,1,12.19,1.66,4.98~~5):}`
Thus, Empirical formula of A si `C_(3)H_(5)` .
`:.` Empirical formul mass = 36 + 5 = 41.
`n = ("Molecular mass")/("Emprirical formula mass")= 83.1/41 = 2.02~~ 2`
Molecular mass is double of empirical formula mass.
`:.` Molecular formula is `C_(6)H_(10)`
To determine the structure of compounds (A) and (B)
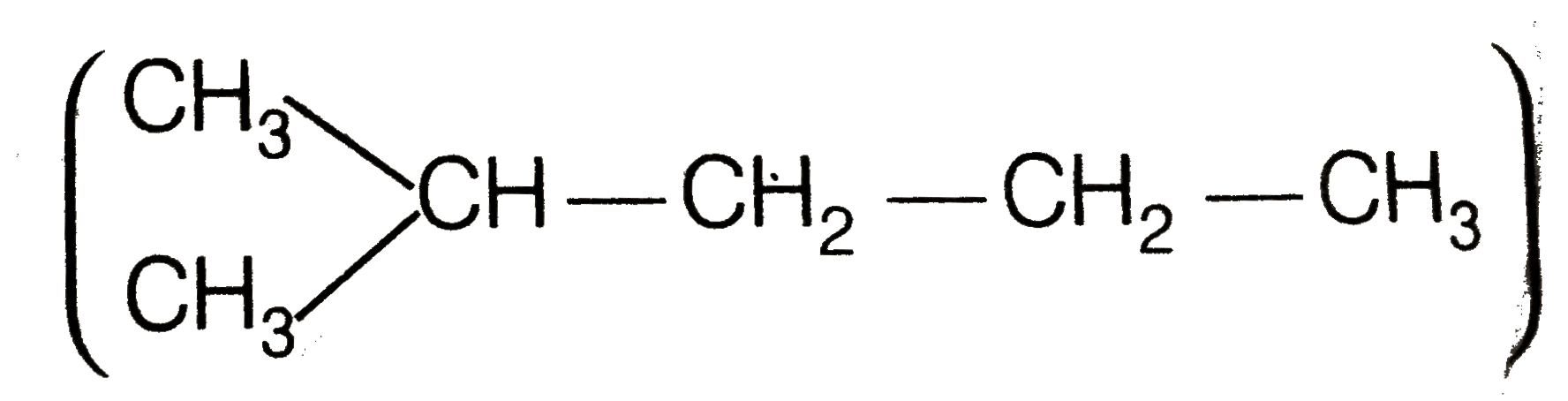
Hence, hydrogenation of hydrocarbon (A) requires 2 moles of hydrogen to form 2-methylpentane. Therefor, hyrocarbon (A) is an alkyne having five carbon atoms in a staight chain and a methyl substituent at position 2. Thus the possible structures for the alkyne (A) are I and II.
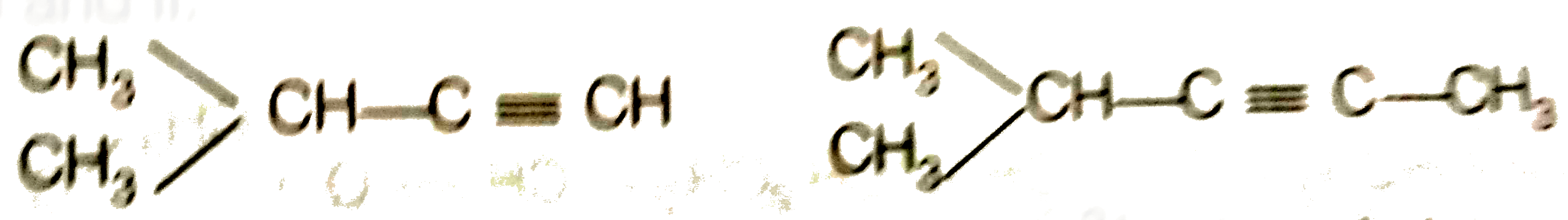
Since, addition of `H_(2)O` to alkyne (A) in presence of `Hg^(2+)` , give a ketong which gives positive iodoform test, therefore, therefore, ketone (B) must be a methyl katon , i.e., it must contain a `COOH_(3)` group.
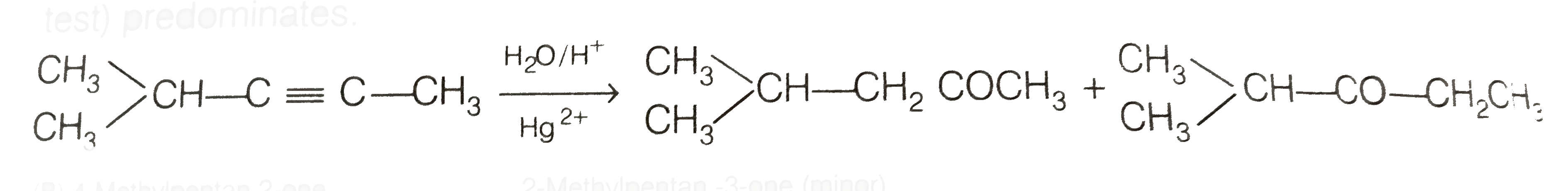
Now addition of `H_(2)O` to alkyne (II) should give a mixture of two ketones in which 2- methyl pentan -3 one (minor) and 4- methylpentan -2-one ketone (B) (which shows `+ve` iodoform test) predominates.
In contrast, addition of `H_(2)O` to alkyne (I) will give only one ketone, i.e., 4-methylpentan-2- one which gives iodoform test.
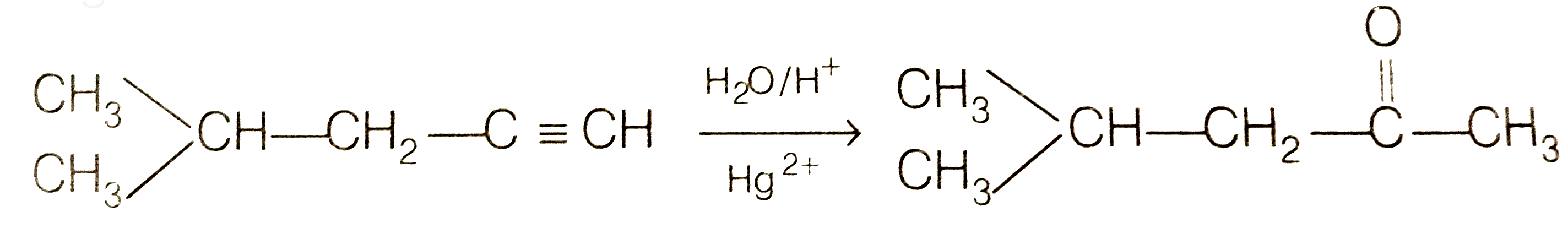
Thus, hydrocabon `C_(x)H_(y)` (A) is 4-methylpent -1-yne. 4-methylpentan -2 one (gives + ve iodoform test)