Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-ALDEHYDE, KETONES AND CARBOXYLIC ACIDS-Aldehyde, Ketones And Carboxylic Acids
- Benzophenone can be obtained by ……
Text Solution
|
- Which of the following is the correct represetation for intermediate o...
Text Solution
|
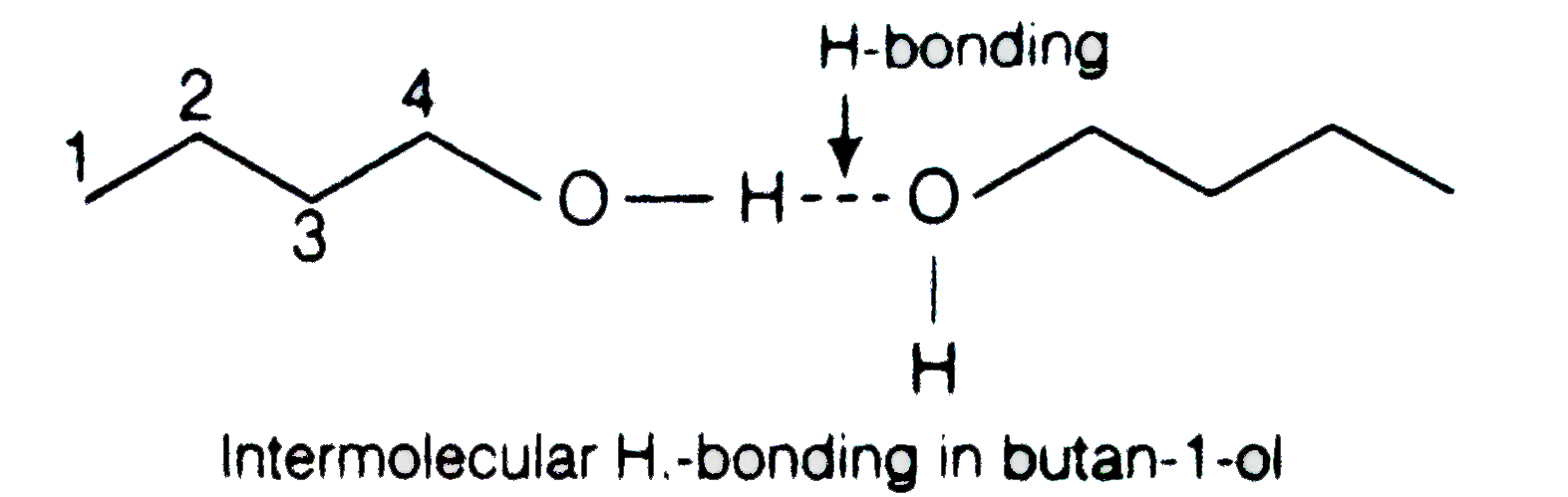
- Why is there a large difference in the boiling points of butanal and b...
Text Solution
|
- Write a test to differentiate between pentan-2-one and pentan-3-one.
Text Solution
|
- Give the IUPAC names of the following compounds. (a) (c) CH(3)-CH...
Text Solution
|
- Give the structure of the following compounds : (i) 4-Nitropropiophe...
Text Solution
|
- Write IUPAC names of the following structures
Text Solution
|
- Benzaldehyde can be obtained from benzalchloride. Write reactions for ...
Text Solution
|
- Name the electrophile produced in the reaction of benzene with benzoyl...
Text Solution
|
- Oxidation of ketones involves carbon-carbon bond cleavage. Name the pr...
Text Solution
|
- Arrange the following in decreasing order of their acidic strength and...
Text Solution
|
- What product will be formed on reaction of propanal with 2-methylpropa...
Text Solution
|
- Compound 'A' is prepared by oxidation of compound 'B' with alkaline KM...
Text Solution
|
- Arrange the following in decreasing order of their acidic strength. Gi...
Text Solution
|
- Alkenes and carbonyl compounds both contain a pi bond but alkenes sh...
Text Solution
|
- Carboxylic acids contain carbonyl group but do not show the nucleophil...
Text Solution
|
- Identify the compounds A, B and C in the following reaction : CH(3)-...
Text Solution
|
- Why are carboxylic acids more acidic than alcohols or phenols although...
Text Solution
|
- Complete the following reaction sequence : CH(3)-overset(O)overset(|...
Text Solution
|
- Ethylbenzene is generally prepared by acetylation of benzene followed ...
Text Solution
|