Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-ALDEHYDE, KETONES AND CARBOXYLIC ACIDS-Aldehyde, Ketones And Carboxylic Acids
- Alkenes and carbonyl compounds both contain a pi bond but alkenes sh...
Text Solution
|
- Carboxylic acids contain carbonyl group but do not show the nucleophil...
Text Solution
|
- Identify the compounds A, B and C in the following reaction : CH(3)-...
Text Solution
|
- Why are carboxylic acids more acidic than alcohols or phenols although...
Text Solution
|
- Complete the following reaction sequence : CH(3)-overset(O)overset(|...
Text Solution
|
- Ethylbenzene is generally prepared by acetylation of benzene followed ...
Text Solution
|
- Can Gattermann-Koch reaction be considered similar to Friedel Craft's ...
Text Solution
|
- Match the common names given in Column I with the IUPAC names given in...
Text Solution
|
- Match the acids given in Column I with their correct IUPAC names given...
Text Solution
|
- Match the reactions given in Column I with the suitable reagents given...
Text Solution
|
- Match the example given in Column I with the name of the reaction in C...
Text Solution
|
- Assertion (A) Formaldehyde is a planar molecule. Reason (R) It conta...
Text Solution
|
- Assertion (A) compound containing -CHO group are easily oxidised to co...
Text Solution
|
- Assertion (A) The alpha-hydrogen atom in carbonyl compounds is less ac...
Text Solution
|
- Assertion : Aromatic aldehydes and formaldehyde undergo Cannizzaro rea...
Text Solution
|
- Assertion (A) Aldehydes and ketones, both react with Tollen's reagent ...
Text Solution
|
- An alkene 'A' (molecular formula C(5)H(10)) on ozonolysis gives a mixt...
Text Solution
|
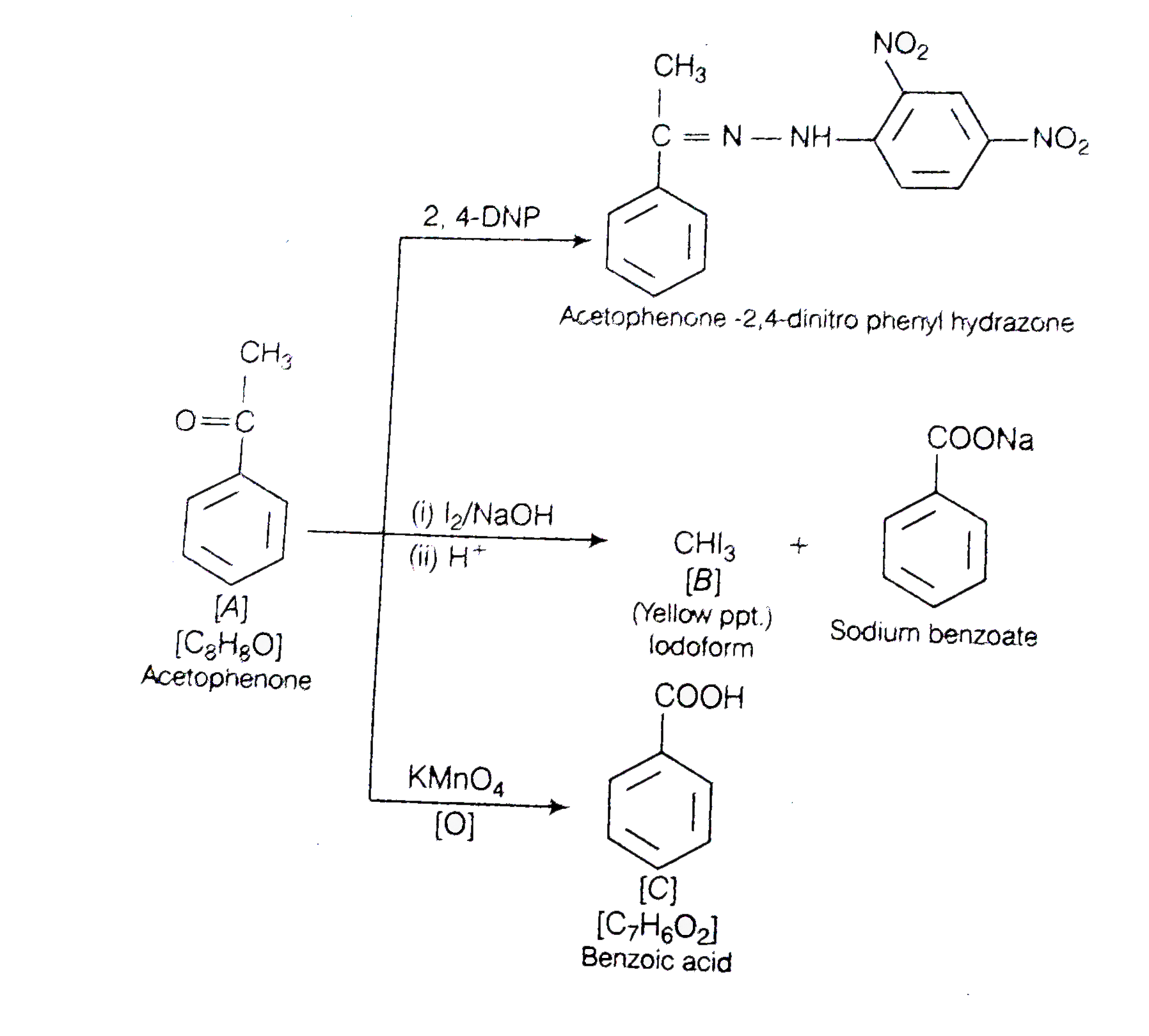
- An aromatic compound 'A' (Molecular formula C(8)H(8)O)) gives positive...
Text Solution
|
- Write down functional isomers of a carbonlyl compound with molecular f...
Text Solution
|
- When liquid 'A' is treated with a freshly prepared ammoniacal silver n...
Text Solution
|