Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-CHEMISTRY IN EVERYDAY LIFE-Chemistry In Everyday Life
- Hair shampoos belong to which class of synthetic detergent ?
Text Solution
|
- Dishwashing soaps are synthetic detergents. What is their chemical nat...
Text Solution
|
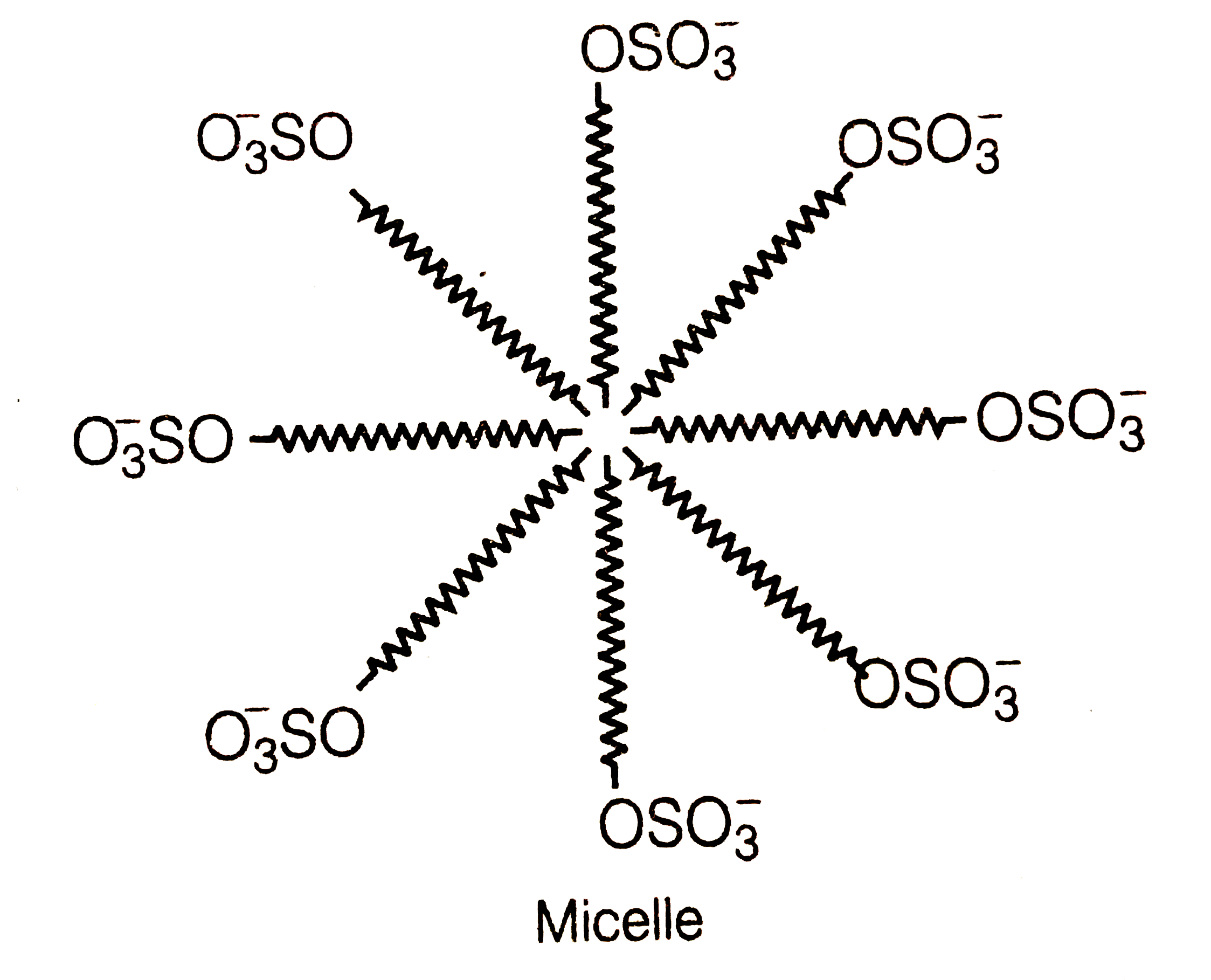
- Draw the diagram showing micelle formation by the following detergen...
Text Solution
|
- How does the branching of hydrocarbon chain of synthetic detergents a...
Text Solution
|
- Why is it safer to use soap from the enviromental point of view ?
Text Solution
|
- What are analgesics ?
Text Solution
|
- What is the scientific explanation for the feeling of depression ?
Text Solution
|
- What is the basic difference between antiseptics and disinfectant...
Text Solution
|
- Between sodiumhydrogen carbonate and magnesium hydroxide , which i...
Text Solution
|
- Which anaglesis are called opiates ?
Text Solution
|
- What is the medicinal use of narcotic drugs ?
Text Solution
|
- What are antagonistric drug ?
Text Solution
|
- What is the mode of action of antimicrobial drugs?
Text Solution
|
- What is the side product of soap industry ? Give reactions showing so...
Text Solution
|
- What is the differene between bathing soap and washing soaps ?
Text Solution
|
- How are transparent soaps manufactured ?
Text Solution
|
- What is the advantage of using antihistamines over antacids in the tre...
Text Solution
|
- What are the functions performed by histamine in the body ?
Text Solution
|
- With the help of an example explain how do transquilizers control the...
Text Solution
|
- Why are certain drugs called enzyme inhibitors ?
Text Solution
|