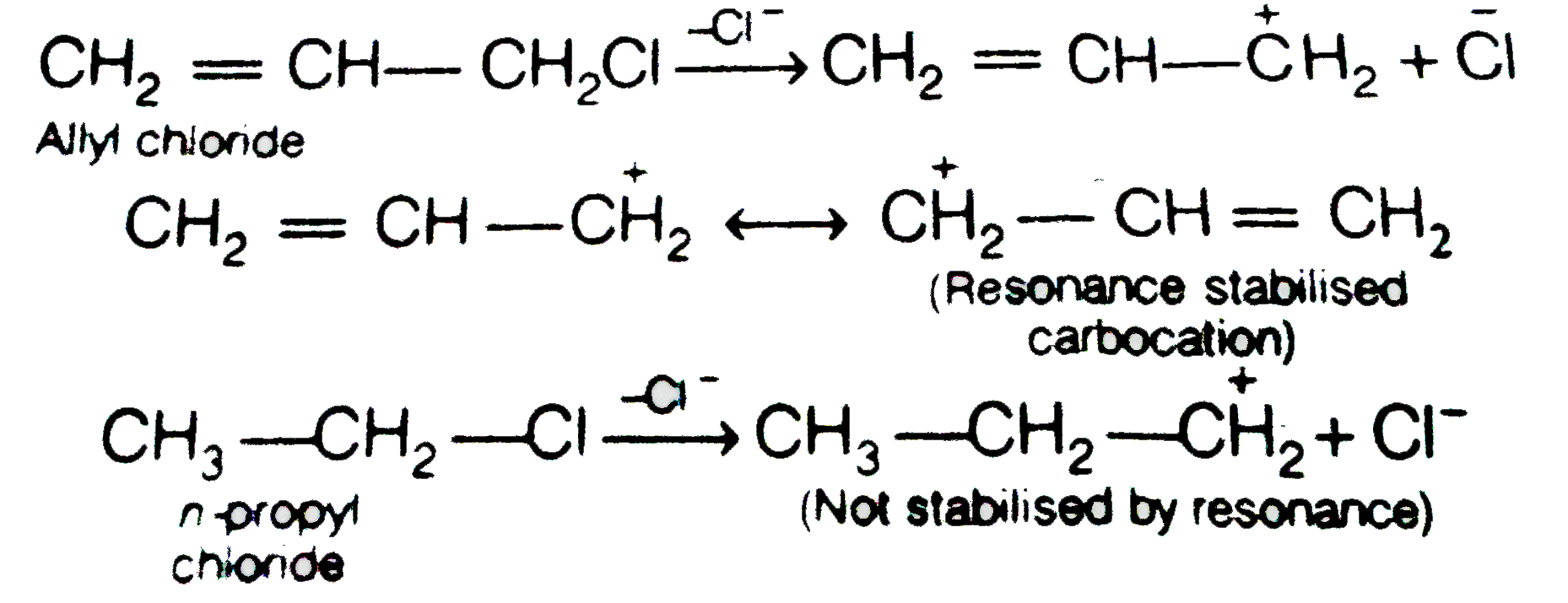Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-HALOALKANES AND HALOARENES-Haloalkanes And Haloarenes
- Why can aryl halides not be prepared by reaction of phenol with HCl in...
Text Solution
|
- Which of the following compounds would undergo SN(1) reaction faster a...
Text Solution
|
- Allyl chloride is hydrolysed more readily than n-propyl chloride. Why ...
Text Solution
|
- Why is it necessary to avoid even traces of moisture during the use of...
Text Solution
|
- How do polar solvents help in the first step in S(N)1 mechanism?
Text Solution
|
- Write a test to detect the presence of double bond in a molecule.
Text Solution
|
- Diphenyls are potential threat to the envioronment. How are these prod...
Text Solution
|
- What are the IUPAC names of the insecticide DDT and benzene hexachlori...
Text Solution
|
- Elimination reaction (especially beta - elimination) are as common as ...
Text Solution
|
- How will you obtain monobromobenzene from aniline ?
Text Solution
|
- Aryl halides are extermely less reactive towards nucleophilic substitu...
Text Solution
|
- tert-Butylbromide reacts with aq. NaOH by S(N)1 mechanism while n-buty...
Text Solution
|
- Predict the major product formed when HCl is added to isobutylene, Exp...
Text Solution
|
- Discuss the nature of C-X bond in the haloarenes.
Text Solution
|
- How can you obtain iodoethane from ethanol when no other iodine contai...
Text Solution
|
- Cyanide ion acts as an ambident nucleophille. From which end it acts a...
Text Solution
|
- Match the compounds given in column I with the effects given in Column...
Text Solution
|
- Match the items of Column I and Column II
Text Solution
|
- Match the structures of compounds given in Column I with the classes o...
Text Solution
|
- Match the reactions given in Column I with the types of reactions give...
Text Solution
|