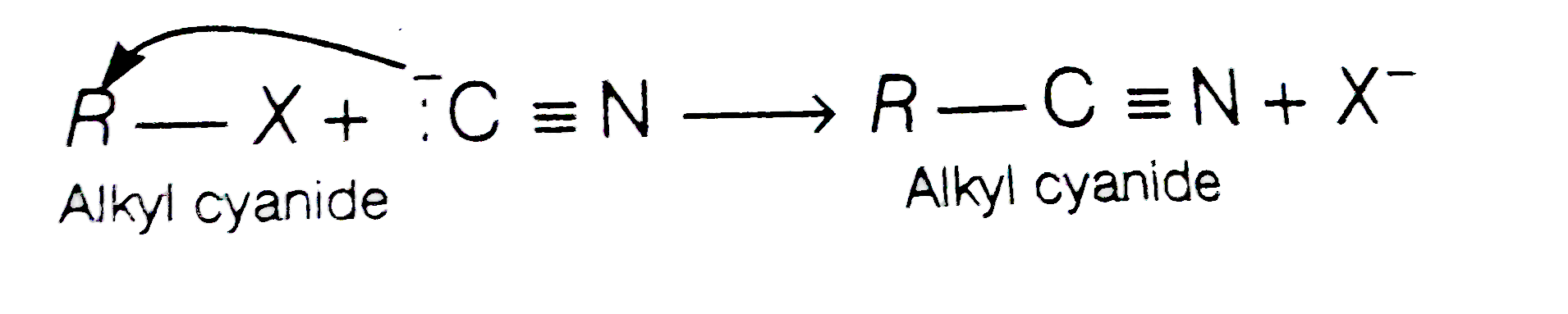Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-HALOALKANES AND HALOARENES-Haloalkanes And Haloarenes
- Discuss the nature of C-X bond in the haloarenes.
Text Solution
|
- How can you obtain iodoethane from ethanol when no other iodine contai...
Text Solution
|
- Cyanide ion acts as an ambident nucleophille. From which end it acts a...
Text Solution
|
- Match the compounds given in column I with the effects given in Column...
Text Solution
|
- Match the items of Column I and Column II
Text Solution
|
- Match the structures of compounds given in Column I with the classes o...
Text Solution
|
- Match the reactions given in Column I with the types of reactions give...
Text Solution
|
- Match the structures given in Column I with the names in Column II
Text Solution
|
- Match the reactions given in Column I with the names givn in Column II...
Text Solution
|
- Assertion ( A) Phosphorus chlorides ( tri and penta) are preferred ove...
Text Solution
|
- Assertion( A) The boiling points of alkyl halides decrease in the orde...
Text Solution
|
- Assertion( A) KCN reacts with methyl chloride to give methyl isocyanid...
Text Solution
|
- Assertion (A) tert-butyl bromide undergoes Wurtz reaction to give 2,2,...
Text Solution
|
- Assertion (A) Presence of a nitro group at ortho or para position incr...
Text Solution
|
- Assertion: In monohaloarenes, further electrophilic substitution occur...
Text Solution
|
- Assertion: Aryl iodides can be prepared by reaction of arenes with iod...
Text Solution
|
- Assertion: It is difficult to replace chlorine by -OH in chlorobenzene...
Text Solution
|
- Assertion (A) Hydrolysis of (-)-2-bromooctane proceeds with inversion ...
Text Solution
|
- Assertion (A) Nitration of chlorobenzene leads to the formation of m-n...
Text Solution
|
- Some alkyl halides undergo substitutionn whereas some undergo eliminat...
Text Solution
|