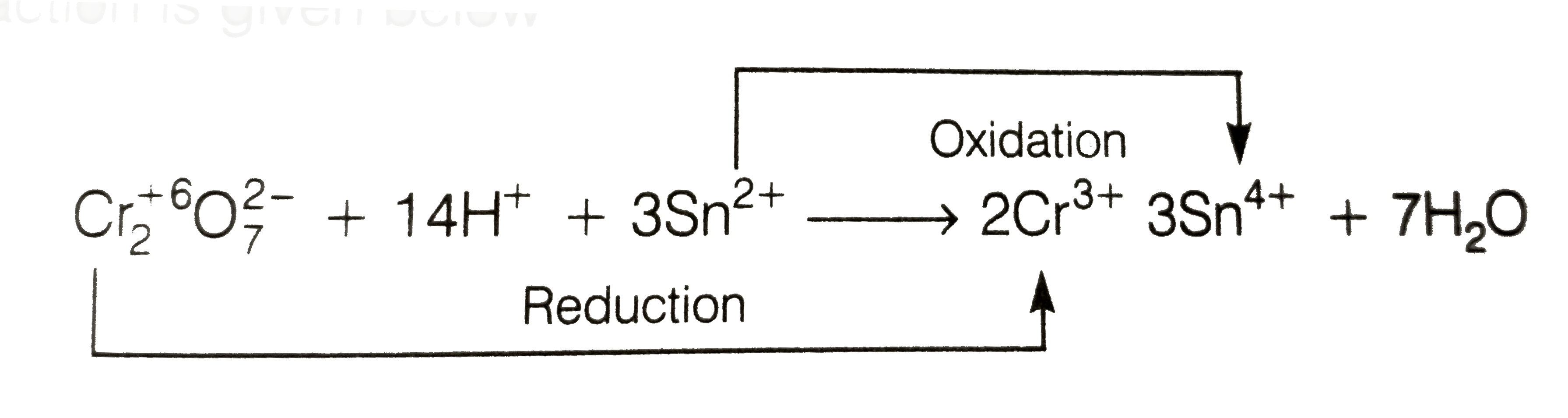
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-D AND F-BLOCK ELEMENTS-D And F-Block Elements
- KMnO(4) acts as on oxidising agent in alkaline medium. When alkaline K...
Text Solution
|
- Which of the following statements is not correct?
Text Solution
|
- When acidified K(2)Cr(2)O(7) solution is added to Sn^(2+) salts then S...
Text Solution
|
- Higher oxidation state of manganese in fluoride is +4 (MnF(4)) but hig...
Text Solution
|
- Although zirconium belongs to 4d transition series and hafnium to 5d t...
Text Solution
|
- Why HCl not used to make the mdeium acidic in oxidation reactions of K...
Text Solution
|
- Generally transition elements and their salts are coloured due to the ...
Text Solution
|
- Transition elements show magnetic moment due to spin and orbital motio...
Text Solution
|
- In the form of dichromate, Cr(VI) is a strong oxidising agent in acidi...
Text Solution
|
- Which of the following actinoids show oxidation states upto +7?
Text Solution
|
- General electronic configuration of actinoids is (n-2)f^(1-14)(n-1)d^(...
Text Solution
|
- Which of the following lanthanoids show +2 oxidation state besides the...
Text Solution
|
- Which of the following ions show higher spin only magnetic moment va...
Text Solution
|
- Transition elements form binary compounds with halogens. Which of the ...
Text Solution
|
- Which of the following will not act as oxidising agents?
Text Solution
|
- Although +3 is the characteristic oxidation state for lanthanoids but ...
Text Solution
|
- Why does copper not replace hydrogen from acids?
Text Solution
|
- Why E^(-) values for Mn, Ni and Zn are more negative than expected?
Text Solution
|
- Why first ionisation enthalpy of Cr is lower than that of Zn?
Text Solution
|
- Transition elements show high melting points. Why?
Text Solution
|