Text Solution
Verified by Experts
Topper's Solved these Questions
THERMODYNAMICS
NCERT EXEMPLAR|Exercise SHORT ANSWER TYPE QUESTIONS|6 VideosTHERMODYNAMICS
NCERT EXEMPLAR|Exercise LONG ANSWER TYPE QUESTIONS|5 VideosTHERMODYNAMICS
NCERT EXEMPLAR|Exercise MULTIPLE CHOICE QUESTIONS (MORE THAN ONE OPTIONS)|5 VideosTHERMAL PROPERTIES OF MATTER
NCERT EXEMPLAR|Exercise Very short Answer type Questions|15 VideosUNITS AND MEASUREMENTS
NCERT EXEMPLAR|Exercise Long Answer Type Questions|9 Videos
Similar Questions
Explore conceptually related problems
NCERT EXEMPLAR-THERMODYNAMICS -VERY SHORT ANSWER TYPE QUESTIONS
- Can a system be heated and its temperature ramains constant ?
Text Solution
|
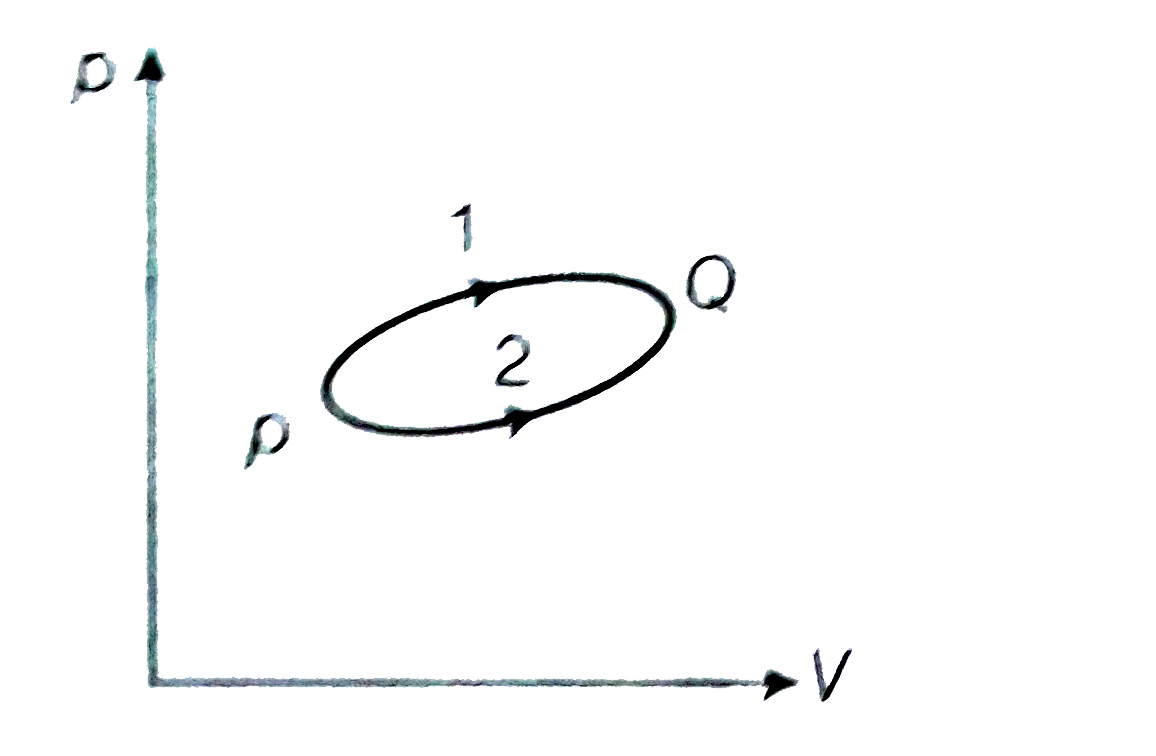
- A system goes from P to Q by two different paths in the p-V diagram as...
Text Solution
|
- If a refrigerator's door is kept open, will the room become cool or ho...
Text Solution
|
- Is it possible in increase the temperature of a gas without adding hea...
Text Solution
|
- Air pressure in a car tyre increase during. Explain.
Text Solution
|