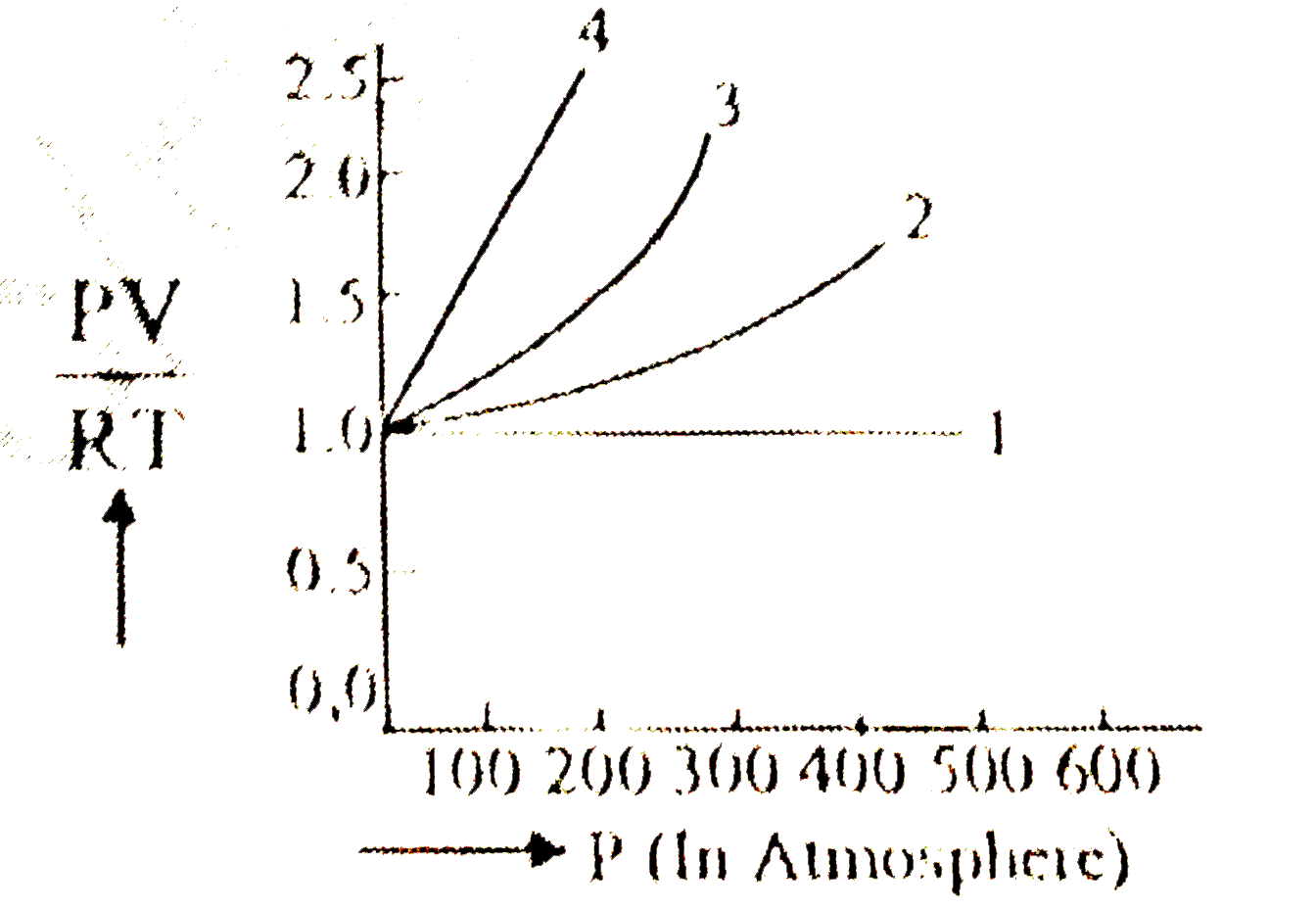A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CAREER POINT-MOCK TEST 10-PART (B) - CHEMISTRY
- A fixed amount of ideal gas (1 mole) is taken and is subjected to pres...
Text Solution
|
- Borate form green colour flame when burunt With (Conc. H(2)S0(4) + Eth...
Text Solution
|
- At STP, a container has 1 mole of Ar, 2 mole of CO(2), 3 moles of O(2)...
Text Solution
|
- The oxidation potential of a hydrogne electrode at pH=10 and p(H(2))=1...
Text Solution
|
- On reduction with hydrogen , 3.6 g of an oxide of metal left 3.2 g of ...
Text Solution
|
Text Solution
|
Text Solution
|
- The back side attack on -- bromobutan by methoxide (CH(3)O^(-)) give...
Text Solution
|
- Major product of this reaction is :
Text Solution
|
- The species having tetrahedral shape is
Text Solution
|
- Electronic configuration of some elements are given: A: 1s^(2) 2s^(2...
Text Solution
|
- Which of the following can react with both HCl and NaOH ?
Text Solution
|
- Which of the following solid has maximum melting points?
Text Solution
|
- The catalyst used in the manufacture of polyethylene Zeigler method is...
Text Solution
|
- The product C is -
Text Solution
|
- Iodine is powerful antiseptic. It is used as a tincture of iodine whic...
Text Solution
|
- Two elemets X( atomic weight =75) and Y( atomic weight =16) combine to...
Text Solution
|
- The value of the spin only magnetic moment for one of the following co...
Text Solution
|
- An element X (At,wt = 80 g//mol) having fcc structure, calculate the n...
Text Solution
|
- Which of the following donot give Cannizzaro reaction ?
Text Solution
|
- Consider the following reactions, The end product 'S' is -
Text Solution
|