A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Thermodynamics
MOTION|Exercise EXERCISE - 2|40 VideosThermodynamics
MOTION|Exercise EXERCISE - 3 SECTION - A|49 VideosThermodynamics
MOTION|Exercise Solved Example|10 VideosSURFACE TENSION & VISCOSITY
MOTION|Exercise Exercise - 4 | Level-II Previous Year | JEE Advanced|12 VideosUNIT & DIMENSIONS
MOTION|Exercise Exercise - 4 (Level - II) (PREVIOUS YEAR JEE ADVANCED)|10 Videos
Similar Questions
Explore conceptually related problems
MOTION-Thermodynamics-EXERCISE - 1
- In the indicator diagram shown, the work done along path AB is
Text Solution
|
- In the indicator diagram shown, In the above problem work done al...
Text Solution
|
- In the indicator diagram shown, In the above question, the work do...
Text Solution
|
- A thermodynamic system is taken through the cycle abcda (a) Calculate...
Text Solution
|
- During the adiabatic change of ideal gas, the realation between the pr...
Text Solution
|
- The slope of indicator curve in adiabatic change relative to volume ax...
Text Solution
|
- The initial volume and pressure of a gas are V and P respectively. It ...
Text Solution
|
- Dry air at one atmospheric pressure is suddenly compressed so that its...
Text Solution
|
- In an adiabatic process
Text Solution
|
- During adiabatic compression of a gas, its temperature
Text Solution
|
- A perfect gas is compressed adiabatically. In that state the value of ...
Text Solution
|
- In an adiabatic expansion of 2 moles of a gas, the change in its inter...
Text Solution
|
- The pressure and volume of a gas are P and V. If its pressure is reduc...
Text Solution
|
- Monatomic diatomic and triatomic gases whose initial volume and pressu...
Text Solution
|
- Two samples of a gas initially at same temperature and pressure are co...
Text Solution
|
- A gas at 105 Pascal pressure and 27ºC temperature is compressed adiaba...
Text Solution
|
- An ideal gas undergoes the process 1 to 2 as shown in the figure, the ...
Text Solution
|
- The pressure and density of a diatomic gas (gamma=7//5) change adiabat...
Text Solution
|
- A gas at NTP is suddenly compressed to one-fourth of its original vol...
Text Solution
|
- In the following P–V diagram of an ideal gas, two adiabates cut two is...
Text Solution
|
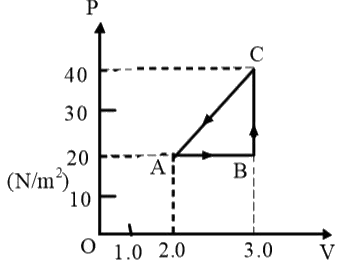 In the above question, the work done along path CA is –
In the above question, the work done along path CA is –