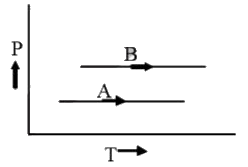
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Thermodynamics
MOTION|Exercise EXERCISE - 3 SECTION - A|49 VideosThermodynamics
MOTION|Exercise EXERCISE - 3 SECTION|1 VideosThermodynamics
MOTION|Exercise EXERCISE - 1|55 VideosSURFACE TENSION & VISCOSITY
MOTION|Exercise Exercise - 4 | Level-II Previous Year | JEE Advanced|12 VideosUNIT & DIMENSIONS
MOTION|Exercise Exercise - 4 (Level - II) (PREVIOUS YEAR JEE ADVANCED)|10 Videos
Similar Questions
Explore conceptually related problems
MOTION-Thermodynamics-EXERCISE - 2
- An ideal gas expands from state (P(1), V(1)) to state (P(2), V(2)), wh...
Text Solution
|
- Consider the two process on a system as shown in figure. The volumes i...
Text Solution
|
- A gas expands in a piston-cylinder device from volume V(1) to V(2), th...
Text Solution
|
- A sample of an ideal gas is expanded to twice its original volume of 1...
Text Solution
|
- There are two parts of a vessel. The pressure in one part is P and its...
Text Solution
|
- A gas is contained in a metallic cylinder fitted with a piston.The pis...
Text Solution
|
- The figure shows the graph of logarithmic reading of pressure and volu...
Text Solution
|
- Two identical containers A and B with frictionless pistons contain the...
Text Solution
|
- A monoatomic ideal gas, initially at temperature T1, is enclosed in a ...
Text Solution
|
- An ideal gas expands isothermally from volume V(1) to V(2) and is then...
Text Solution
|
- Statement-1: The total translational kinetic energy of fall the molecu...
Text Solution
|
- In a thermodynamic process two moles of a monatomic ideal gas obeys PV...
Text Solution
|
- A certain amount of an ideal monatomic gas needs 20 J of heat energy t...
Text Solution
|
- The pressure P of an ideal diatomic gas varies with its absolute tempe...
Text Solution
|
- The variation of pressure P with volume V for an ideal diatomic gas is...
Text Solution
|
- The specific heat of a gas in a polytropic process is given by-
Text Solution
|
- Work done in the cyclic process shown in figure is-
Text Solution
|
- A P-T graph is shown for a cyclic process. Select correct statement re...
Text Solution
|
- One mole of monatomic gas is brought from state A to state B. The init...
Text Solution
|
- One mole of monatomic gas is brought from state A to state B. The init...
Text Solution
|