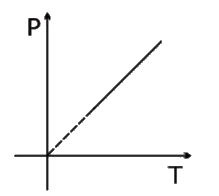
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Thermodynamics
MOTION|Exercise EXERCISE - 3 SECTION - A|49 VideosThermodynamics
MOTION|Exercise EXERCISE - 3 SECTION|1 VideosThermodynamics
MOTION|Exercise EXERCISE - 1|55 VideosSURFACE TENSION & VISCOSITY
MOTION|Exercise Exercise - 4 | Level-II Previous Year | JEE Advanced|12 VideosUNIT & DIMENSIONS
MOTION|Exercise Exercise - 4 (Level - II) (PREVIOUS YEAR JEE ADVANCED)|10 Videos
Similar Questions
Explore conceptually related problems
MOTION-Thermodynamics-EXERCISE - 2
- In a thermodynamic process two moles of a monatomic ideal gas obeys PV...
Text Solution
|
- A certain amount of an ideal monatomic gas needs 20 J of heat energy t...
Text Solution
|
- The pressure P of an ideal diatomic gas varies with its absolute tempe...
Text Solution
|
- The variation of pressure P with volume V for an ideal diatomic gas is...
Text Solution
|
- The specific heat of a gas in a polytropic process is given by-
Text Solution
|
- Work done in the cyclic process shown in figure is-
Text Solution
|
- A P-T graph is shown for a cyclic process. Select correct statement re...
Text Solution
|
- One mole of monatomic gas is brought from state A to state B. The init...
Text Solution
|
- One mole of monatomic gas is brought from state A to state B. The init...
Text Solution
|
- One mole of monatomic gas is brought from state A to state B. The init...
Text Solution
|
- A thermodynamic process is shown in this figure. The pressure and volu...
Text Solution
|
- An ideal gas is taken through the cycle AtoBtoCtoA, as shown in the fi...
Text Solution
|
- In the figure represent cyclic process. The corresponding PV graph is
Text Solution
|
- The figure shows the P-V plot of an ideal gas taken through a cycle AB...
Text Solution
|
- Following figure shows P-T graph for four processes A, B, C and D. Sel...
Text Solution
|
- During the thermodynamic process shown in figure for an ideal gas
Text Solution
|
- During which of the following thermodynamic process represented by PV ...
Text Solution
|
- The variation of pressure P with volume V for an ideal monatomic gas d...
Text Solution
|
- Figure shows, the adiabatic curve on a log T and log V scale performed...
Text Solution
|
- If a gas is taken A to C through B then heat absorbed by the gas is 8 ...
Text Solution
|