A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Thermodynamics
MOTION|Exercise EXERCISE - 3 SECTION|1 VideosThermodynamics
MOTION|Exercise EXERCISE - 3 SECTION b|26 VideosThermodynamics
MOTION|Exercise EXERCISE - 2|40 VideosSURFACE TENSION & VISCOSITY
MOTION|Exercise Exercise - 4 | Level-II Previous Year | JEE Advanced|12 VideosUNIT & DIMENSIONS
MOTION|Exercise Exercise - 4 (Level - II) (PREVIOUS YEAR JEE ADVANCED)|10 Videos
Similar Questions
Explore conceptually related problems
MOTION-Thermodynamics-EXERCISE - 3 SECTION - A
- A refrigerator transfer 180 joule of energy in one second from tempera...
Text Solution
|
- A monatomic gas at a pressure P, having a volume V expands isothermall...
Text Solution
|
- A thermodynamic system undergoes cyclic process ABCDA as shown in figu...
Text Solution
|
- A monatomic gas at a pressure P, having a volume V expands isothermall...
Text Solution
|
- Assertion : For gas atom the number of degrees of freedom is 3. R...
Text Solution
|
- If for hydrogen C(P) - C(V) = m and for nitrogen C(P) - C(V) = n, wher...
Text Solution
|
- A diatomic gas undergoes adiabatic compression and its volume reduces ...
Text Solution
|
- One of the most efficient engines ever developed operated between 2100...
Text Solution
|
- The cofficient of performance of a refrigerator is 5. If the temperatu...
Text Solution
|
- An ideal gas is compressed to half its initial volume by means of seve...
Text Solution
|
- A Carnot engine, having an efficiency of eta= 1/10 as heat engine, is ...
Text Solution
|
- One mole of an ideal diatomic gas undergoes a transition from A to B a...
Text Solution
|
- In (figure). shows two path that may be taken by a gas to go from a st...
Text Solution
|
- A refrigerator works between 4^(@)C and 30^(@)C. It is required to rem...
Text Solution
|
- A gas is compressed isothermally to half its initial volume. The same ...
Text Solution
|
- Thermodynamic processes are indicated in the following diagram. M...
Text Solution
|
- A Carnot engine, having an efficiency of eta= 1/10 as heat engine, is ...
Text Solution
|
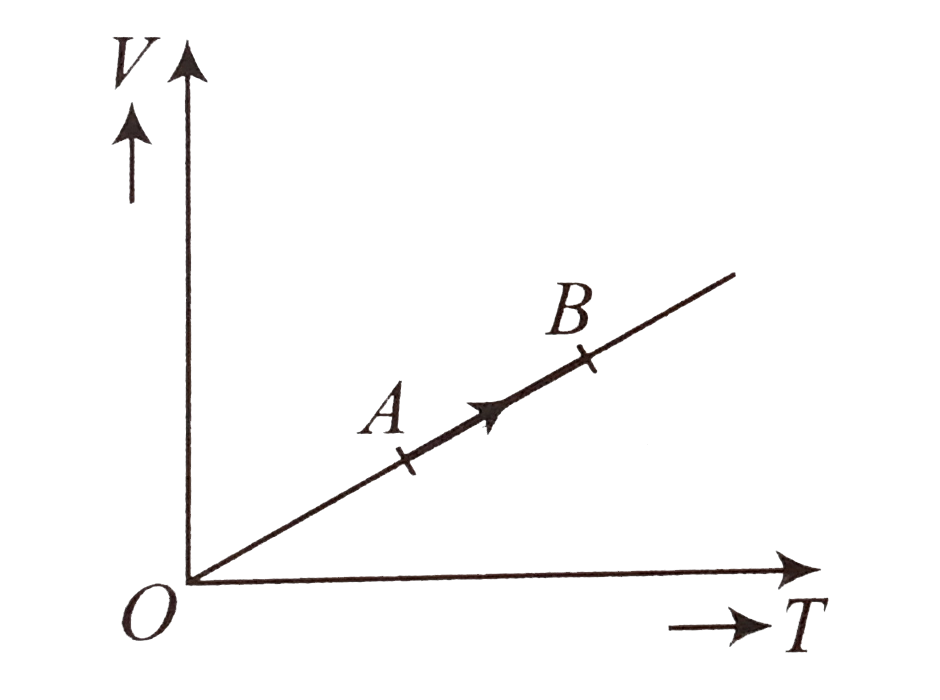
- The volume (V) of a monatomic gas varies with its temperature (T) as s...
Text Solution
|
- The efficiency of an ideal heat engine working between the freezing po...
Text Solution
|
- A sample of 0.1 g of water at 100 and normal pressure (1.013 xx10^(5)...
Text Solution
|