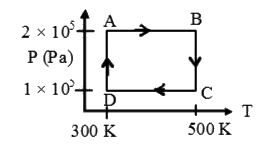
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
MOTION-Thermodynamics-EXERCISE - 3 SECTION b
- Which of the following statements is correct for any thermodynamic sys...
Text Solution
|
- Which of the following is incorrect regarding the first law of thermod...
Text Solution
|
- The temperature -entropy diagram of a reversible engine cycle is given...
Text Solution
|
- A system goes from A and B via two processes. I and II as shown in fig...
Text Solution
|
- The work of 146 kJ is performed in order to compress one kilo mole of ...
Text Solution
|
- A Carnot engine, having an efficiency of eta= 1/10 as heat engine, is ...
Text Solution
|
- When a system is taken from state i to state f along the path iaf, it ...
Text Solution
|
- Assume the gas to be ideal, the work done on the gas in taking it from...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- Two moles of helium gas are taken over the cycle ABCDA, as shown in th...
Text Solution
|
- A diatomic ideal gas is used in a Carnot engine as the working substan...
Text Solution
|
- A thermally insulated vessel contains an ideal gas of molecular mass M...
Text Solution
|
- A carnot engine operating between temperatures T(1) and T(2) has effic...
Text Solution
|
- Helium gas goes through a cycle ABCDA (consisting of two isochoric and...
Text Solution
|
- A Carnot engine, whose efficiency is 40%, takes in heat from a source ...
Text Solution
|
- The above p-v diagram represents the thermodynamic cycle of an engine,...
Text Solution
|
- One mole of a diatomic ideal gas undergoes a cyclic process ABC as sho...
Text Solution
|
- A solid body of constant heat capacity 1J//^@C is being heated by keep...
Text Solution
|
- n' moles of an ideal gas undergoes a process AtoB as shown in the figu...
Text Solution
|
- Two moles of an ideal monatomic gas occupies a volume V at 27 °C. The ...
Text Solution
|