Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-PRACTICE PAPER 1-Questions
- Explain why ozone is thermodynamically less stable then oxgen ?
Text Solution
|
- Write the name of gas released when Cu added to dilute HNO(3)
Text Solution
|
- Cu reacted to Conc. HNO(3), which type of gas released during the rea...
Text Solution
|
- Give one disproportionation reaction of phosphorus acid (H(3)PO(3)).
Text Solution
|
- Draw the structure of XeF(2) molecule.
Text Solution
|
- Account for the following: Although Fluorine has less negative elect...
Text Solution
|
- Account for the following: Acidic character decreases from N(2)O(3) ...
Text Solution
|
- Write a chemical reaction to test sulphur dioxide gas. Write chemical ...
Text Solution
|
- E("Cell") for the given redox reaction is 2.71 V Mg((s))+Cu((0.01 M)...
Text Solution
|
- A steady current of 2 amperes was passed through two electrolytic cell...
Text Solution
|
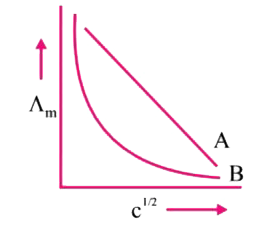
- In the plot of molar conductivity (^ m) vs square root of concentratio...
Text Solution
|
- How do you convert the following : Phenol to Anisole
Text Solution
|
- How will you convert ethanol to propan-2-ol ?
Text Solution
|
- Explain the mechanism of the following reaction: CH(3)CH(2)OH overse...
Text Solution
|
- Why phenol undergoes electrophilic substitution more easily than benze...
Text Solution
|
- o-nitrophenol is more volatile than p-nitrophenol it is due to
Text Solution
|
- Account for the following: t-butyl chloride on heating with sodium m...
Text Solution
|
- Write the reaction involved in the following : Reimer-Tiemann reacti...
Text Solution
|
- Write the reaction involved in the following : Friedal-Crafts Alkyla...
Text Solution
|
- Give simple chemical test to distinuish between Ethanol and Phenol.
Text Solution
|