Text Solution
Verified by Experts
Topper's Solved these Questions
ALDEHYDES,KETONES AND CARBOXYLIC ACIDS
CBSE COMPLEMENTARY MATERIAL|Exercise SHORT ANSWER -II TYPE QUESTIONS (3 MARKS)|44 VideosALDEHYDES,KETONES AND CARBOXYLIC ACIDS
CBSE COMPLEMENTARY MATERIAL|Exercise LONG ANSWER TYPE QUESTIONS (5 MARKS)|21 VideosALDEHYDES,KETONES AND CARBOXYLIC ACIDS
CBSE COMPLEMENTARY MATERIAL|Exercise VERY SHORT ANSWER TYPE QUESTIONS (1 MARK)|19 VideosALCOHOLS, PHENOLS AND ETHERS
CBSE COMPLEMENTARY MATERIAL|Exercise LONG ANSWER TYPE QUESTIONS (5 Marks)|5 VideosAMINES
CBSE COMPLEMENTARY MATERIAL|Exercise 5 MARKS|7 Videos
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-ALDEHYDES,KETONES AND CARBOXYLIC ACIDS-SHORT ANSWER -I TYPE QUESTIONS (2 MARKS)
- Give the chemical test of distinguish between : CH(3)CHO and CH(3)-ove...
Text Solution
|
- Give the chemical test of distinguish between : CH(3)CHO and C(6)h(5)C...
Text Solution
|
- Would you expect benzaldehyde to be more reactive or less reactive in ...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger ? ...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger ? C...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger ? C...
Text Solution
|
- Which acid of each pair shown here would you expect to be stronger ? .
Text Solution
|
- Carboxylic acids do not give reactions of aldehydes and ketones why ?
Text Solution
|
- Write IUPAC name of the following : .
Text Solution
|
- Write IUPAC name of the following : .
Text Solution
|
- Accout for the following : (i) Oxidation of toluence to C(6)H(5)CHO wi...
Text Solution
|
- Accout for the following : (ii) Melting point of an acid with even num...
Text Solution
|
- Distinguish between : (i) C(2)H(5)OH and CH(3)CHO.
Text Solution
|
- Distinguish between : (ii) C(6)H(5)COCH(3) and C(6)H(5)CH(2)CHO.
Text Solution
|
- Complete the following reactions by identitying A: A+ "Hydrogen" (g)...
Text Solution
|
- Compelte the following reactions by identifying B and C: (ii) CH(3)-ov...
Text Solution
|
- Benzaldehyde gives a positive test with Tollen's reagent but not with ...
Text Solution
|
- Aldehydes usually do not form stable hydrates but chloral normally exi...
Text Solution
|
- Give possible explanation for the following : (i) Cyclohexanone forms ...
Text Solution
|
- Give possible explanation for the following : (ii) There are two - NH2...
Text Solution
|
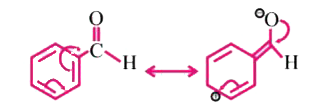 .
.