Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-ANNUAL EXAM.-2018-19-Questions
- A ball is thrown vertically upwards with a velocity of 20 ms^(-1) from...
Text Solution
|
- The flow rate of water from a tap of diameter 1.25 cm is 0.48 L//mi n....
Text Solution
|
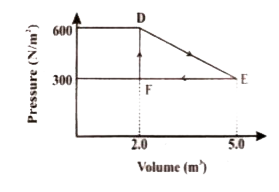
- A thermodynamic system is taken from original state D to an intermedia...
Text Solution
|
- What is total internal reflection of light ? What are conditions for t...
Text Solution
|
- A particle starts from origin at t=0 with velocity 5.0hati m//s and mo...
Text Solution
|
- Consider a simple pendulum having a bob attached to a string that osci...
Text Solution
|
- State work-energy theorem. Prove it for a variable force.
Text Solution
|
- A saturn year is 29.5 times the earth year. How far is the saturn from...
Text Solution
|
- Find out the position of centre of mass of two particle system.
Text Solution
|
- State Kepler's laws of planetary motion.
Text Solution
|
- Define orbital velocity of a nearest satellite revolving around the ea...
Text Solution
|
- Obtain following equation from first principles: (i) w=w(0+a t (...
Text Solution
|
- Derive an expression for the work done in an isothermal process.
Text Solution
|
- State law of equipartition of energy. Using this law, determine the va...
Text Solution
|
- Derive Newton's formula for speed of sound in an ideal gas. What is La...
Text Solution
|
- A transverse harmonic wave on a string is described by y(x, t)=3.0 sin...
Text Solution
|
- Derive an expression for the time -period of the horizontal oscillatio...
Text Solution
|
- Define angle of friction and angle of repose. Show that both are numer...
Text Solution
|
- A body tied to one end of a string is made to revolve in a vertical ci...
Text Solution
|
- Define viscosity and write SI unit of coefficient of viscosity.
Text Solution
|