Text Solution
Verified by Experts
Topper's Solved these Questions
CBSE EXAMINATION PAPER - 2016
CBSE COMPLEMENTARY MATERIAL|Exercise SECTION - E|17 VideosCBSE EXAMINATION PAPER - 2016
CBSE COMPLEMENTARY MATERIAL|Exercise SECTION - B|5 VideosCBSE BOARD EXAMNATION PAPER (2017 )
CBSE COMPLEMENTARY MATERIAL|Exercise SECTION E|12 VideosCBSE Examination Paper Delhi–2014
CBSE COMPLEMENTARY MATERIAL|Exercise SET-III (Questions Uncommon to set-I and II)|9 Videos
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-CBSE EXAMINATION PAPER - 2016 -SECTION - C
- A charge is distributed uniformly over a ring of radius ‘a’. Obtain an...
Text Solution
|
- Write three characteristic features in photoelectric effect which cann...
Text Solution
|
- Write the expression for the magnetic force acting on a, charged parti...
Text Solution
|
- A neutron, an electron an alpha particle moving equal velocities, inte...
Text Solution
|
- Define mutual inductance.
Text Solution
|
- A pair of adjacent coils has a mutual inductance of 1.5. H. If the cur...
Text Solution
|
- Two parallel plate capacitors X and Y have the same area of plates and...
Text Solution
|
- Two parallel plate capacitors X and Y have the same area of plates and...
Text Solution
|
- Two parallel plate capacitors X and Y have the same area of plates and...
Text Solution
|
- How are electromagnetic waves produces by oscillating charges ? Draw a...
Text Solution
|
- Write Maxwell’s generalization of Ampere’s Circuital Law. Show that in...
Text Solution
|
- Calculate the distance of an object of height h from a concave mirror ...
Text Solution
|
- Using mirror formula, explain why does a convex mirror always produce ...
Text Solution
|
- (i) State Bohr's quantization condition for defining stationary ...
Text Solution
|
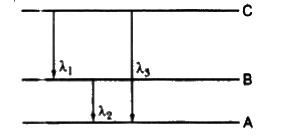
- Find the relation between the three wavelengths lamda1, lamda2and lam...
Text Solution
|
- Draw a schematic ray diagram of reflecting telescope showing how...
Text Solution
|