Text Solution
Verified by Experts
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
CBSE COMPLEMENTARY MATERIAL-ELECTRONIC DEVICES -NUMERICALS
- In a p-n junction, width of depletion region is 300 nm and electric fi...
Text Solution
|
- An LED is constructed from a p-n junction based on a certain semi-cond...
Text Solution
|
- Determine V(0) and I(d) for the network.
Text Solution
|
- A p-n junction is fabricated from a semiconductor with band gap of 2.8...
Text Solution
|
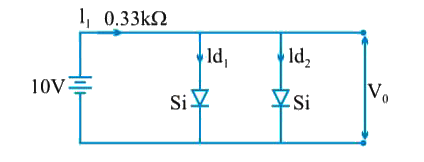
- Determine V(0), I(d1) and I(d2) for the given network. Where D(1) and ...
Text Solution
|
- Pure Si at 300 K has equal electron (n(e)) and hole (n(h)) concentrati...
Text Solution
|
- The solar radiation spectrum shows that maximum solar intensity is nea...
Text Solution
|