A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-JEE MOCK TEST 26-CHEMISTRY
- Which one is the wrong statement ?
Text Solution
|
- When MnO(2) is fused with KOH, a coloured compound is formed. The prod...
Text Solution
|
- A metal is illumimated by light of two different wavelength 248nm and ...
Text Solution
|
- The number and type of bonds between two carbon atoms in CaC(2) are:
Text Solution
|
- For the reaction: X(2)O(4)(l)rarr2XO(2)(g) DeltaU=2.1 cal , DeltaS...
Text Solution
|
- A balloon filled with oxygen is placed in a tank full of hydrogen gas ...
Text Solution
|
- In the Hall-Heroult process for the extraction of Al, which of the fol...
Text Solution
|
- Which of the following acts as an oxidising as well as reducing agent ...
Text Solution
|
- Determine the order of stability of the following resonating structure...
Text Solution
|
- The ionization energies of Li and Na are 520 kJ mol ^-1 and 495 kJ mol...
Text Solution
|
- In the following reaction sequence, structures of P and Q , are respec...
Text Solution
|
- Provide the systematic name of the compound shown
Text Solution
|
- Which reagent can be used to convert a carboxylic acid chloride into a...
Text Solution
|
- Which of the following can not be made by reduction of ketone or alden...
Text Solution
|
- which of the following statement is correct for the reactivity in S(N)...
Text Solution
|
- pH of the anodic solution of the following cell is Pt, H2(1atm)|H^+(xM...
Text Solution
|
- The vapour pressure of pure water at 26^@C is 25.5 torr. . The vapour...
Text Solution
|
- The number of geometric isomers of the complex Cr(NH3)3Cl3 are
Text Solution
|
- A hydrocarbon (A) CnH(2n-4) on ozonolysis gives (CH3)2CHCH2CHO, 2 OH...
Text Solution
|
- The gas phase decomposition of dimethylether follows first order kinet...
Text Solution
|
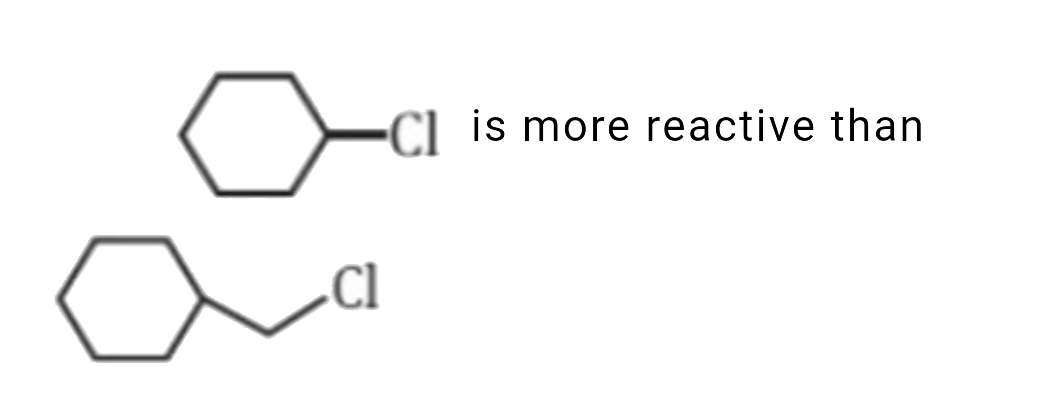
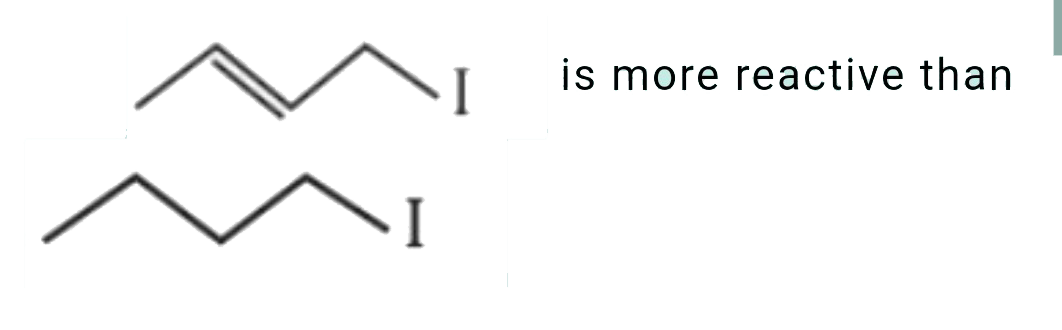
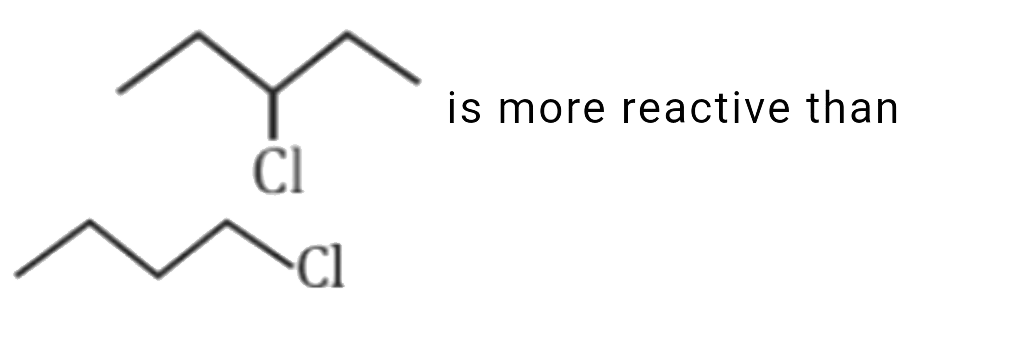
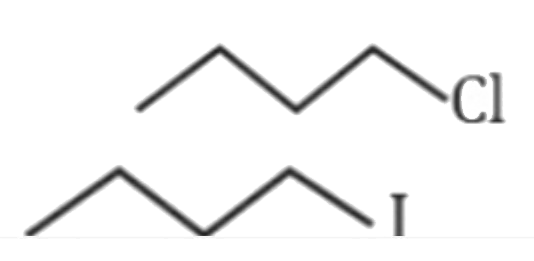 is more reactive than
is more reactive than