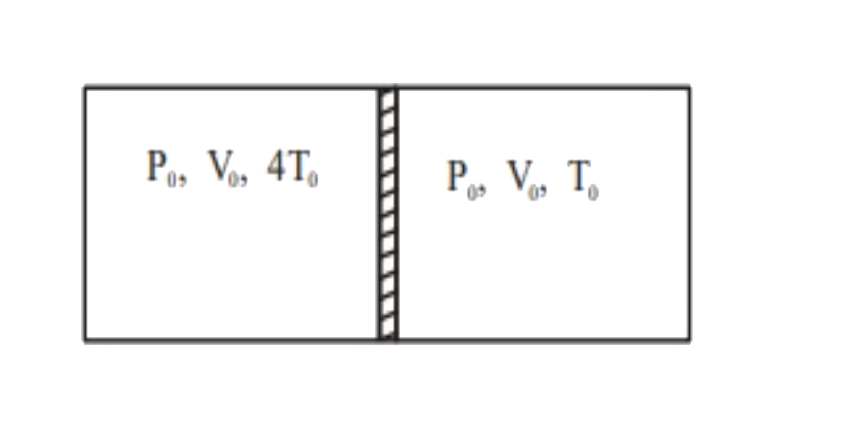A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 27-PHYSICS
- Two spherical conductors A and B of radii R and 2R respectively , are ...
Text Solution
|
- The figure shows a current - carrying square loop ABCD of side 10 cm a...
Text Solution
|
- A block of mass 5 kg is placed on a rough inclined plane. The inclinat...
Text Solution
|
- A block of mass m & charge q is released on a long smooth inclined pla...
Text Solution
|
- A cylindrical adiabatic container of total volume 2V(0) divided into t...
Text Solution
|
- The pressure at the bottom of an open tank of water is 3p where p is t...
Text Solution
|
- The elastic limit of brass is 379 MPa . What should be the minimum dia...
Text Solution
|
- A disc of radius R rolls without slipping at speed v along positive x-...
Text Solution
|
- A vernier callipers has 20 divisions on the vernier scale which coinci...
Text Solution
|
- Radiation of wavelength lamda is incident on a photocell. The fastest ...
Text Solution
|
- In the figure shown , RB=500kOmega, RC = 8 kOmega, V("BB")=10.6V and V...
Text Solution
|
- The figure shows a fixed inclined plane on which a plank is being pull...
Text Solution
|
- A thin prism of angle 5^(@)is placed at a distance of10cm from object....
Text Solution
|
- A micture of light, consisting of wavelength 590nm and an unknown wave...
Text Solution
|
- A particle is released from a height S. At certain height its kinetic ...
Text Solution
|
- Total number of elements which are present in a row on the periodic ta...
Text Solution
|
- A diamtomic molecule can be modelled as two rigid balls connected with...
Text Solution
|
- A body of mass 3 kg collides elastically with another body at rest and...
Text Solution
|
- A man in a balloon throws a stone downwards with a speed of 5 m//s wi...
Text Solution
|
- A block of mass 1 kg is connected with a smooth plank of the same mass...
Text Solution
|