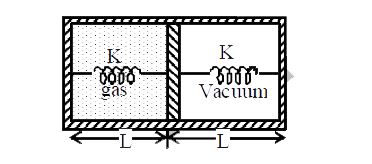
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 31-PHYSICS
- Two conducting spheres of radii r(1) and r(2) having charges Q(1) and ...
Text Solution
|
- A large hollow metal sphere of radius R has a small opening at the to...
Text Solution
|
- A body is projected upwards with a velocity of 4 xx 11.2 "km s"^(-1) f...
Text Solution
|
- When heat is supplied to the gas it expands and displaces piston by L/...
Text Solution
|
- Two coaxial long solenoids of equal length have current, i(1), i(2), n...
Text Solution
|
- A wedge of mass m, lying on a rough horizontal plane, is acted upon by...
Text Solution
|
- The length of a simple pendulum executing simple harmonic motion is in...
Text Solution
|
- A 27 mW laser beam has a cross-sectional maximum electric field ...
Text Solution
|
- The level of water in a tank is 5 m high . A hole of the area 10 cm^2 ...
Text Solution
|
- A wire suspended vertically from one of the its ends is stretched by a...
Text Solution
|
- The maximum fequency for reflection of sky waves from a certain layer ...
Text Solution
|
- 1% of 10^12 Hz of a satellite link was used for telephony . What is th...
Text Solution
|
- The wave of wavelength 5900 Å emitted by any atom or molecule must hav...
Text Solution
|
- A light string is tied at one end to a fixed support and to a heavy st...
Text Solution
|
- A particle starts with speed v(0) from x = 0 along x - axis with retar...
Text Solution
|
- A ring of mass 4 kg is uniformaly charged with a linear charge density...
Text Solution
|
- An object P is kept in front of two plane mirrors as shown in the figu...
Text Solution
|
- Four solid sphereas each of diameter sqrt(5) cm and mass 0.5 kg are pl...
Text Solution
|
- Two ideal monoatomic and diatomic gases are mixed with one another to ...
Text Solution
|
- The density of a sphere is measured by measuring the mass and diameter...
Text Solution
|