A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 35-CHEMISTRY
- For the reaction 5Br^(-)(aq)+BrO(3)^(-)(aq)+6H^(+)(aq)rarr 3Br(2)(aq...
Text Solution
|
- Polyvinyl alcohol is an important polymer. The structure is given belo...
Text Solution
|
Text Solution
|
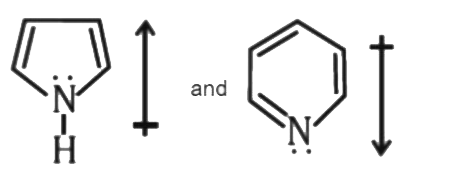
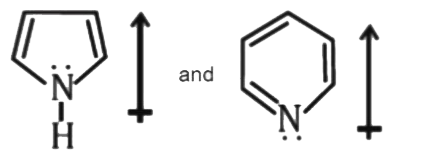
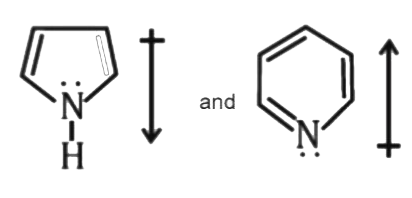
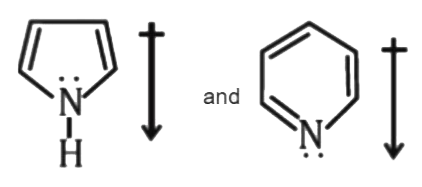
- The correct orientation of dipoles in pyrrole and pyridine is
Text Solution
|
- Upon long standing concetrated HNO(3)
Text Solution
|
- In which of the following crystals alternate tetrahedral voids are occ...
Text Solution
|
- Which of the following represents correcty the changes in thermodynami...
Text Solution
|
- What represents the best method for converting a carboxylic acid to an...
Text Solution
|
- Aluminum carbide (Al(4)C(3)) liberates methane on treatment with water...
Text Solution
|
- Give the major product of the following reaction
Text Solution
|
- Which of the following is correct?
Text Solution
|
- The van der Waals equation for one mole of a real gas can be written a...
Text Solution
|
- For the reaction 2CO(g)+O(2)(g)rarr 2CO(2), DeltaH=-500kJ. Two mol...
Text Solution
|
- There are four complexes of Ni. Select the complexes/es which will be ...
Text Solution
|
- A mixture of three alkyl chloride CH(3)Cl, CH(3)CH(2)Cl, CH(3)CH(2)CH(...
Text Solution
|
- A cell contains two hydrogen electrodes. The negative electrode is in ...
Text Solution
|
- The number of compounds in which complete delocalisation of pi- electr...
Text Solution
|
- Among the following metals how many metals are extracted by self-reduc...
Text Solution
|
- In the esterification C(2)H(5)OH(l)+CH(3)COOH(l)" an "hArr CH(3)COOC...
Text Solution
|
- The number of unbranched isomers (including stereoisomers) of C(6)H(12...
Text Solution
|