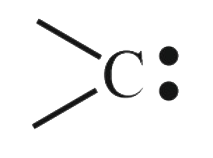
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 47-CHEMISTRY
- The smallest ketone and its next homologue are reacted with NH(2)OH to...
Text Solution
|
- The minimum voltage required to electrolyse alumina in the Hall-Herout...
Text Solution
|
- Which one of the following mixture does not act as a buffer solution?
Text Solution
|
- The enthalpy of neutralization of NH(4)OH and CH(3)COOH is – 10.5 kcal...
Text Solution
|
- Lattice energy of an ionic compound depedns upon :
Text Solution
|
- Which product will be obtained by Gridnard reaction, when Formaldehyde...
Text Solution
|
- Which of the following represents the correct order of increasing firs...
Text Solution
|
- Compound that is both paramagnetic and coloured is:
Text Solution
|
- The reactant A is
Text Solution
|
- Out of the following, which is the correct match for the radial probab...
Text Solution
|
- The rate of decomposition of NH(3) on platinum surface is zero order. ...
Text Solution
|
- In which of the solution hydrogen peroxide neither acts as oxidising a...
Text Solution
|
- The intermediate never formed during chain growth polymerization is
Text Solution
|
- The product A is
Text Solution
|
- The density of KBr is 2.75 g cm^(-3) length of the unit cell is 654 p ...
Text Solution
|
- Calculate the number of hours of service that can be derived at 1 atm,...
Text Solution
|
- The difference in the number of unpaired electrons in Co^(2+) ion in i...
Text Solution
|
- How many among the following species can be classified as Lewis acids?...
Text Solution
|
- What weight of glucose dissolved in 100g of water will produce the sam...
Text Solution
|
- A 1.0 M solution of Cd^(2+) is added to excess iron and the system is ...
Text Solution
|