A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 49-CHEMISTRY
- An acid-base indicator has a K(a) of 3.0 xx 10^(-5). The acid form of ...
Text Solution
|
- Among the following, the number of reaction(s) that produce(s) benzal...
Text Solution
|
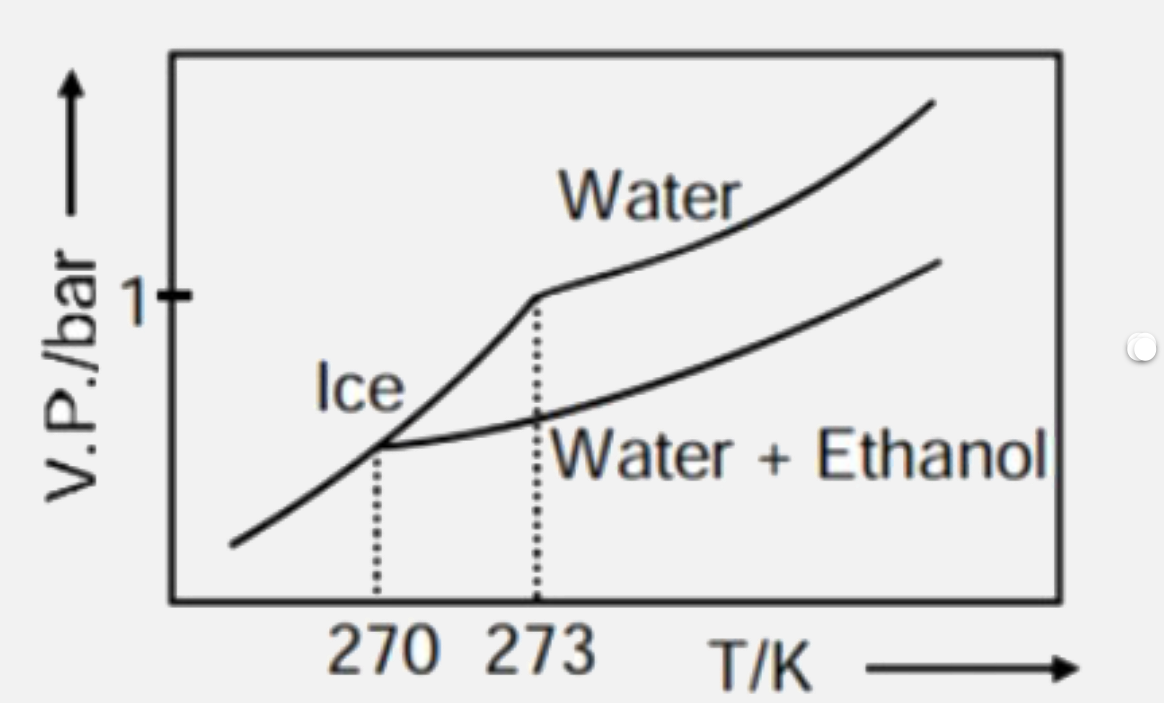
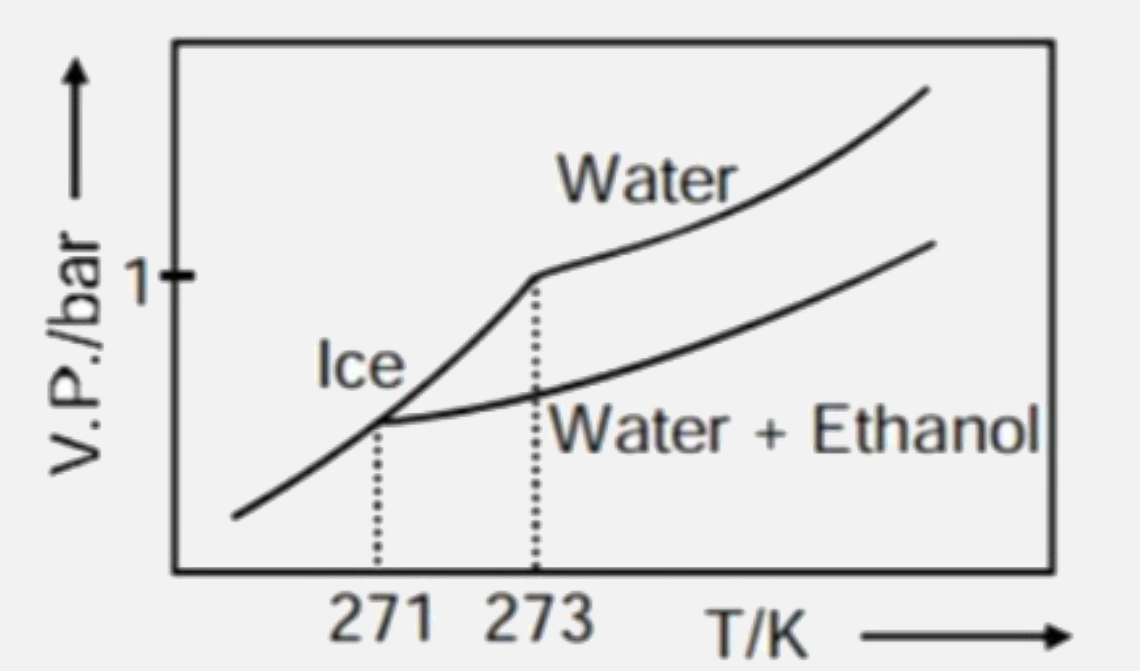
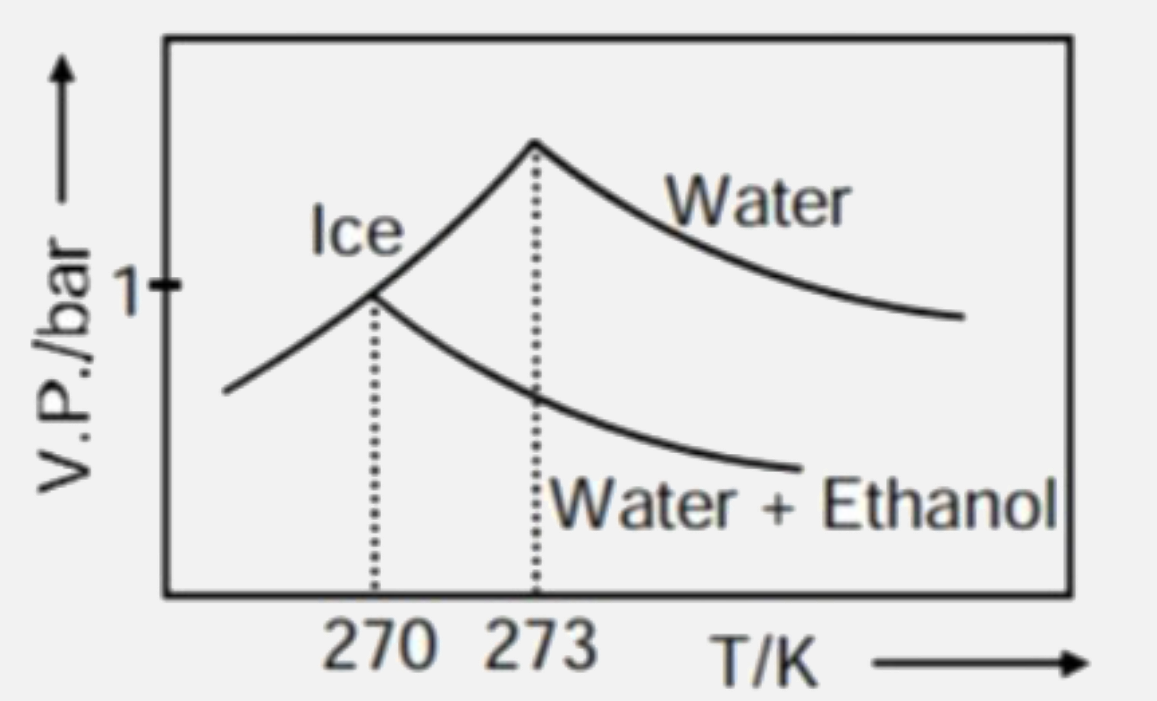
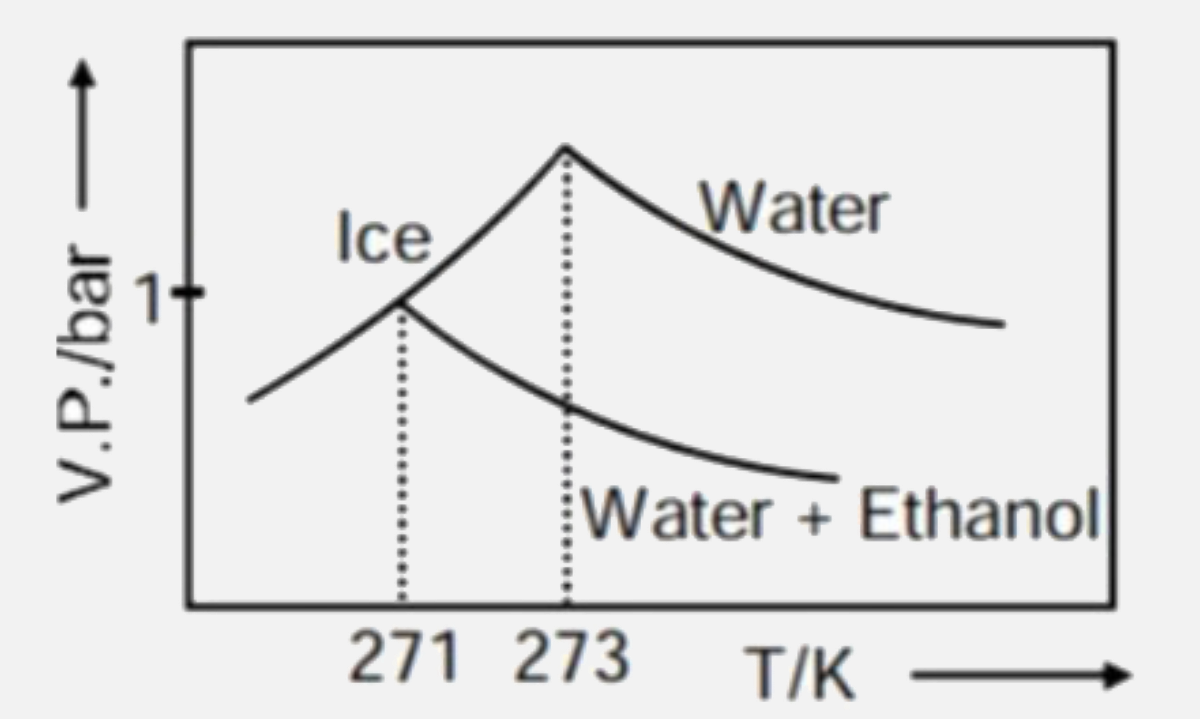
- Pure water freezes at 273 K and 1 bar. The addition of 34.5 g of ethan...
Text Solution
|
- Which of the following atoms has the highest first ionisation energy ?
Text Solution
|
- For the following cell, Zn(s)|ZnSO(4)(aq)||CuSO(4)(aq)||Cu(s) When...
Text Solution
|
- Which of the following products can be formed when 2 -chloro -2- methy...
Text Solution
|
- In the following reaction, the major product is
Text Solution
|
- Which of the following gives ketone on oxidation?
Text Solution
|
- Among [Ni(CO)(4)], [NiCl(4)]^(2-), [Co (NH(3))(4) Cl(2)] Cl, Na(3) [C...
Text Solution
|
- Which of the following combination will produce H(2) gas ?
Text Solution
|
- Given that for a reaction of nth order, the integrated rate equation i...
Text Solution
|
- Which of the following is not correctly matched?
Text Solution
|
- The major product of the following reaction is
Text Solution
|
- Which of the following disaccharide will not reduce Tollen's reagent?
Text Solution
|
- The concentration of fluoride, lead, nitrate and iron in a water sampl...
Text Solution
|
- The sum of the number of lone pairs of electrons on each central atom ...
Text Solution
|
- Among the following, the number of aromatic compound(s) is
Text Solution
|
- Three moles of B(2)H(6) are completely reacted with methanol. The numb...
Text Solution
|
- A crystalline solid of a pure substance has a face-centred cubic struc...
Text Solution
|
- Equilibrium constant for reaction NH(4)OH(aq)+H^(+)(aq)hArr NH(4)^(+)(...
Text Solution
|