A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 53-CHEMISTRY
- For which of the following van't Hoff factor cannot be greater than un...
Text Solution
|
- The number of isomers (including stereoisomers) of C5H(10) are
Text Solution
|
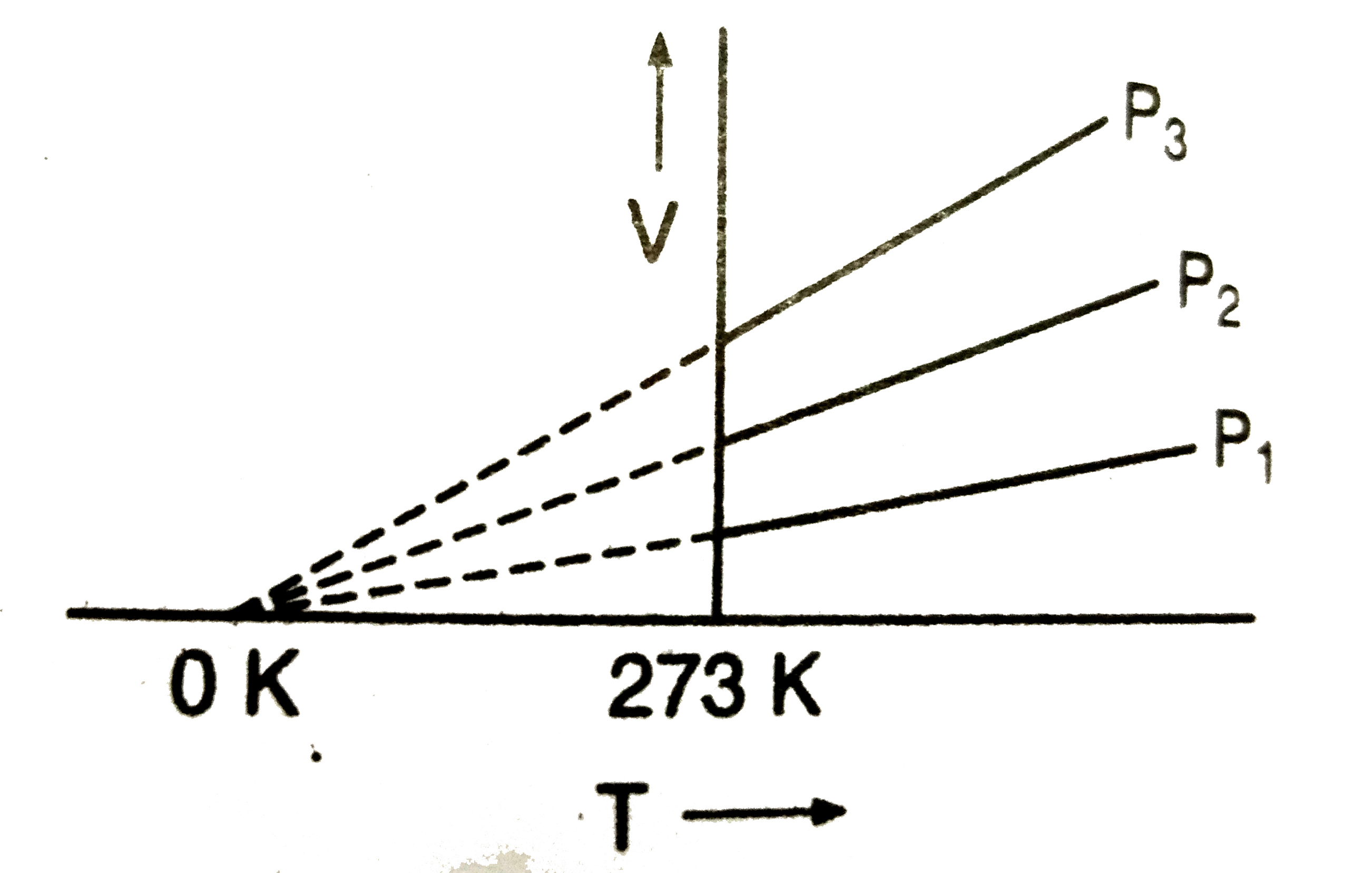
- The volume-temperature graphs of a given mass of an ideal gas at const...
Text Solution
|
- SO(2) turns into which acid when mixed with water
Text Solution
|
- A reaction is 50% complete in 2 hours and 75% complete in 4 hours. Wha...
Text Solution
|
- Total number of geometrical isomers for the complex [Rh Cl (CO) (PPh(3...
Text Solution
|
- In case of nitrogen, NCl(3) is possible but not NCl(5) while in case o...
Text Solution
|
- The lattice energy of solid NaCl is 180K. Cal mol^(-1). The dissolutio...
Text Solution
|
- Which of the following explain the poling process?
Text Solution
|
- Salicylic acid is produced when phenol in alcoholic KOH is treated wit...
Text Solution
|
- Consider the following radial distribution function diagrams. Which of...
Text Solution
|
- In the following sequence of reactions the products D is HC-=CH over...
Text Solution
|
- The compound B is : CH(3)CH(2)COOH overset(Cl(2))underset(red P)rarr...
Text Solution
|
- The two forms of D-glucopyranose obtained from solution of D-glucose a...
Text Solution
|
- The equilibrium constant for the given reaction is approximately 10^(-...
Text Solution
|
- Order of K(a) will be :
Text Solution
|
- Among CaH(2),NH(3), and B(2)H(6) which are covalent hydrides?
Text Solution
|
- The most common oxidation states of cerium are
Text Solution
|
- Which of the following can give iodoform test? (I) CH(3)-overset(O)o...
Text Solution
|
- An elemental crystal has density of 8570 kg m^(-3). The packing effici...
Text Solution
|