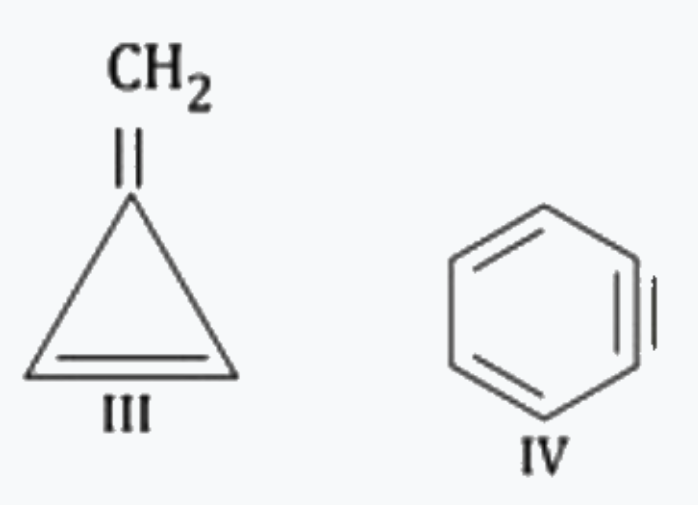
A
B
C
D
Text Solution
Verified by Experts
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 55-CHEMISTRY
- The major product of the following reaction is :
Text Solution
|
- Why is SiCl(4) readily hydrolysed but C Cl(4) is resistant to hydrolys...
Text Solution
|
- Which of the following arrangements shows the schematic alignment of m...
Text Solution
|
- The order of stability of the following tautomeric compound is under...
Text Solution
|
- The following reactions show the H(2)O(2) behaviour in A and B reactio...
Text Solution
|
- The effective nuclear charge of an element with three valence electron...
Text Solution
|
- [P]overset(Br(2))rarrC(2)H(4)Br(2)overset(NaNH(2))rarrQ overset(20%H(2...
Text Solution
|
- What will be the expression of K(p) for the given reaction if the tota...
Text Solution
|
- Mole fraction of component A in vapour phase is chi(1) and that of com...
Text Solution
|
- Which of the following structure contains sp - hybridized carbon atoms...
Text Solution
|
- Identify 'M' in the following sequence of reactions underset((M))(C(...
Text Solution
|
- What are 'X' and 'Y' respectively?
Text Solution
|
- The voltage of the cell consisting of Li((s)) and F(2(g)) electrodes i...
Text Solution
|
- When AgNO(3) solution is added in excess to 1 M solution of CoCl(3).XN...
Text Solution
|
- What will be the nature of existence of an amino acid (containing one ...
Text Solution
|
- At 400 K, the root mean square (rms) speed of a gas X (molecular weigh...
Text Solution
|
- The total number of carboxylic acid groups in the product P is
Text Solution
|
- If the formula of basic Beryllium nitrate is [Be(n)O(NO(3))(6)]. What ...
Text Solution
|
- How many of these metals can show +3 oxidation state also. Sc, Ti, V...
Text Solution
|
- Amongst the following, the total number of compounds soluble in aqueou...
Text Solution
|