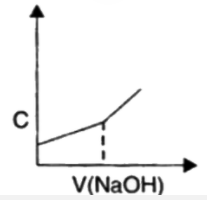
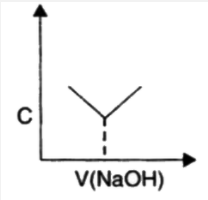
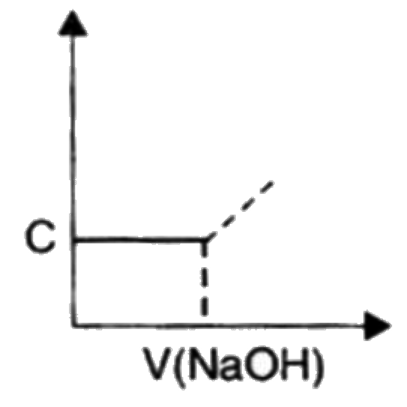
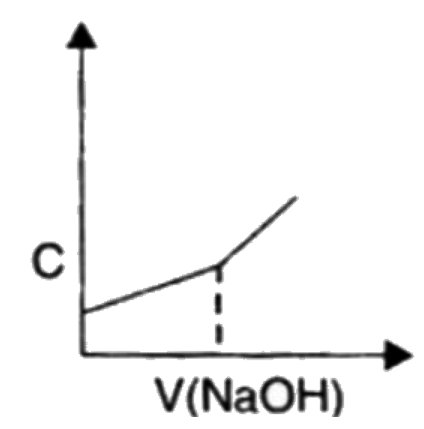
A
B
C
D
Text Solution
AI Generated Solution
The correct Answer is:
Topper's Solved these Questions
Similar Questions
Explore conceptually related problems
NTA MOCK TESTS-NTA JEE MOCK TEST 58-CHEMISTRY
- The specific rotation of a pure enantiomer is +10^(@). The observed ro...
Text Solution
|
- A certain reaction occurs in two steps as (I) 2SO(2(g))+2NO(2(...
Text Solution
|
- An ideal gas is expand from (P(1),V(1),T(1)) to (P(2),V(2),T(2)) under...
Text Solution
|
- Which of the following electrolytes will have maximum coagulating valu...
Text Solution
|
- The possible product obtained from the reaction of cyclobutyl amine wi...
Text Solution
|
- Consider the reaction equilibrium, 2SO(2(g)) + O(2(g))hArr SO3 (g), De...
Text Solution
|
- The correct order of reactivity for the addition reaction of the follo...
Text Solution
|
- For the reaction, A+BrarrP,-(d[A])/(dt)=-(d[B])/(dt)=k[A][B] and Rt=...
Text Solution
|
- Which of the following cations has the strongest tendency towards comp...
Text Solution
|
- Which chemical change among the following involves absorption of heat?
Text Solution
|
- Which of the following curve will represent the variation of conductan...
Text Solution
|
- overset(Ca(OH)(2)(aq))rarrAoverset(NH(3)//Delta)underset(Br(2)+NaOH)ra...
Text Solution
|
- The electrode potential, E^(@), for the reduction of MnO(4)^(-)" to "M...
Text Solution
|
- What final product will form when alcoholic KOH is treated with 1, 1-...
Text Solution
|
- Consider the following antibiotics (i) Erythromycin (ii) Ofloxacin...
Text Solution
|
- How many statements is/are correct for given complex [Co(C(2)O(4))(3)]...
Text Solution
|
- The number of alkenes (including stereoisomers) which can produce 2 - ...
Text Solution
|
- The K(sp) of Mg(OH)(2) is 1xx10^(-12). 0.01 M Mg(OH)(2) will precipita...
Text Solution
|
- An organic compound A ( mol wt = 180) is acylated with CH3 COCl to get...
Text Solution
|
- 1.5 gm sample of bleaching power was suspended in water. If was treate...
Text Solution
|